Clinical and Microbiological Effects of Root Debridement on Periodontal Sites with Different Severity Levels
Vivian Barbosa1, Marcia Rejane Thomas Canabarro Andrade2 and Antonio Canabarro3*
1Veiga de Almeida University, Rio de Janeiro, RJ, Brazil
2Fluminense Federal University. Nova Friburgo, RJ, Brazil
3Veiga de Almeida University and State University of Rio de Janeiro, Brazil
Submission: July 21, 2023; Published: August 02, 2023
*Corresponding author: Antonio Canabarro, Veiga de Almeida University and State University of Rio de Janeiro, Brazil. Email id: andradejr13@gmail.com
How to cite this article: Antonio C. Clinical and Microbiological Effects of Root Debridement on Periodontal Sites with Different Severity Levels. Adv Dent & Oral Health. 2023; 16(3): 555937. DOI: 10.19080/ADOH.2023.16.555937
Abstract
Aim: The objective of this study was to evaluate the clinical and microbiological impact of ultrasonic full mouth debridement associated to a single oral hygiene instruction (1-OHI+FMD) on sites of periodontitis patients with different levels of severity.
Methods: In this 3-month split-mouth clinical trial, 20 periodontitis patients contributed with 3 intraoral niches: - PC (control sites with no probing attachsment loss (PAL), PM (moderate sites with PAL= 3-4 mm) and PS (severe sites with PAL≥ 5 mm). All participants presented high levels of plaque before therapy (visible plaque index (VPI)>20%). 1-OHI+FMD included a 10-min oral hygiene instruction followed by a maximum of 2 sessions of ultrasonic debridement.
Results: clinical evaluation revealed a significant decrease of probing pocket depth (PPD, - 0.51 mm; p< 0.001) and PAL (- 0.47 mm; p= 0.002) in PM and in PS (PPD, - 1.08 mm; PAL, - 0.82 mm; p<0.001) after 3 months. Bleeding on probing was also reduced in all patients after therapy (- 26.23%, p<0.001) but not VPI (- 10.4%, p=0.089). Checkerboard DNA-DNA hybridization only showed reduced bacterial counts in PS, including Prevotella intermedia, Prevotella nigrescenses, Tannerella forsythia and Porphyromonas gingivalis after 3 months (p<0.001). Red and Orange complex was also reduced in PS (p<0.001).
Conclusion: 1-OHI+FMD promoted an improvement in some clinical and microbiological parameters of periodontitis’ patients, especially in deep sites, but it did not seem to be effective in improving the patient’s plaque control, regardless of disease severity.
Keywords: Periodontitis; Periodontal Debridement; Bacteria
Introduction
Gingivitis and periodontitis are diseases that affect the gingival and dental support tissues, respectively. While gingivitis is characterized by reversible gingival inflammation, periodontitis causes irreversible attachment loss, resulting in the formation of periodontal pockets and, eventually, tooth loss [1]. Periodontal diseases also exhibit an association with systemic conditions [2]. Periodontal pockets can harbor more than 500 bacterial species that are, for the most part, resident species living as a stable community in a biofilm. However, an imbalance in the microbiota can promote the multiplication of putative pathogens [3] resulting in dysbiosis. [4]. Several studies have shown the release of a great variety of inflammatory mediators because of microbial imbalance, which promotes inflammation and destruction of periodontal tissues [1].
The periodontal microbial communities have been characterized based on a color-coded system that reflects clustering analysis, community ordering, and disease severity [5] The so-called “red complex”, a group of three gram-negative bacterial species, including Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola, were related to inflammatory and clinical periodontal parameters [5]. However, although gram-negative putative pathogens are thought to play a key role in the pathogenesis of periodontal diseases, there seems to be no single etiological agent in inflammatory periodontal diseases [6]. In fact, red complex bacteria can be found in several niches, including clinically healthy sites [6,7]. Then, treatment would be aimed at lowering the numbers of all bacteria to levels compatible with health [6].
Manual removal (or reduction) of calculus and supra- and subgingival biofilm can successfully control gingivitis and periodontitis. Different mechanical therapies have shown the ability to improve clinical parameters, and a quadrant-scaling approach is the gold standard in the treatment of periodontal diseases [8-10]. However, it takes a long time, it is uncomfortable, and the patient´s schedule availability should be considered. In an attempt to reduce time and bacteria recolonization, full-mouth debridement (FMD) has been introduced [11]. On the basis of the best available data, FMD is as good as quadrant scaling and root planing for the treatment of periodontitis [12]. Additional benefits of FMD include systemic effect, stimulus of immunological response and a better cost-benefit approach [12,13] which makes this treatment especially attractive to patients and general dentists. However, the compliance of patients with oral hygiene is often difficult to achieve, [14] especially considering the nonuse of chemical agents. Furthermore, the reduced number of visits to the dentist can hamper the development of abilities to control the biofilm and ultimately compromise motivation. The objective of this study was to evaluate the clinical and microbiological impact of ultrasonic full mouth debridement associated to a single oral hygiene instruction (1-OHI+FMD) on different niches of periodontitis patients.
Material and Methods
In this 3-month split-mouth clinical trial, patients with periodontitis, according to the new classification of periodontal and peri-implant diseases and conditions [15] were submitted to full-mouth debridement. After responding to the anamnesis, the patients signed the informed consent form and were submitted to data collection, which was repeated 3 months after the periodontal treatment. The study was ethically conducted based on the Helsinki Declaration and was approved by the Human Research Ethics Committee under the number 1.436.434. Fifty individuals of both genders aged between 32 and 70 years in dental treatment were initially invited to participate in this study. They had to present periodontitis. In order to characterize periodontitis, the following criteria were used: age over 35 years old, presence of 2 or more teeth with at least 1 observable buccal or interproximal site with a probing attachment loss (PAL) ≥ 3 mm and a probing pocket depth (PPD) > 3 mm [15,16].
The exclusion criteria for the patients were as follows: gingival recession, smokers, presence of diabetes or other systemic diseases, periodontal treatment for at least one year, pregnancy, lactation, use of prostheses, medical conditions that could affect the existence of bacteria in the periodontal tissues (e.g., HIV, antibiotic therapy, or non-steroidal anti-inflammatory drug use for at least six months). All patients underwent a complete periodontal examination by the same periodontist (VS), using a manual periodontal probe (PCPUNC, Hu-Friedy, Chicago, IL). Periodontal examinations included measurements of PAL, PPD, visible plaque index (VPI), and BOP. All parameters were measured in 6 sites (mesiobuccal, buccal, distobuccal, mesiopalatal or mesiolingual, lingual or palatal, and disto-lingual or disto-palatal), except third molars.
After the examination, all patients were assigned to interventions. Three groups related to different intra-oral niches of the same patients were formed:
- PC (control, saliva), PM (moderate sites, PAL= 3-4 mm), and PS (severe sites, PAL≥ 5 mm) 15.
Bacterial examination was defined as the primary outcome. PAL, PPD, VPI, and BOP were secondary outcomes. Examiner calibration was performed during a pilot study that preceded the present investigation. PAL, PPD, BOP, and VPI were carried out by one examiner at different times. Six sites of all present teeth from 10 patients with periodontitis were examined twice with a week interval. The Interclass Correlation Coefficient (ICC) for all clinical parameters must be higher than 0.8.
Bacterial collection was performed as previously described [17]. Briefly, relative isolation was performed with cotton rollers. The teeth were dried, and subgingival biofilm samples were collected using 1 cone of sterile size 45 paper (Dentisply, Rio de Janeiro, Brazil) from 7 sites selected in all patients with periodontitis: PC= 1 (saliva), PM= 3 and PS= 3. The cone remained in the periodontal pocket or sulcus for 30 seconds. For saliva collection, the cone was passed 3 times on the floor of the mouth. The same sites were sampled again after the periodontal therapy (3 months). The cone with collected material was immediately deposited in individual plastic tubes and stored under refrigeration at -20 °C until the samples were analyzed by the DNA-DNA hybridization checkerboard technique for bacterial strains [17]. Counts and proportions of 40 bacterial species were determined in each sample by using the checkerboard DNA–DNA hybridization technique as shown in a previous study [17].
a) Treatment Protocol: all patients underwent full-mouth debridation by an experienced periodontist (VS). Periodontal therapy (1-OHI+FMD) included a single oral hygiene instruction (10 minutes), tooth brushing and interdental plaque control by dental floss, followed by supra- and subgingival debridement which was performed by ultrasonic scaler tips (Jet Sonic, Gnatus, Rio de Janeiro, Brazil) under local anesthesia when requested by patients. The number of visits was limited to two with a one-day interval. Mean instrumentation time was 25 min per quadrant.
The oral hygiene instructions were not reinforced during follow-up of patients.
After 3 months, a new periodontal examination and microbiological collection were performed in the same teeth and sites previously evaluated.
Statistical evaluation was performed using the SPSS 17.0 program (IBM, New York, NY). Initially, the data distribution was verified with the Kolgomorov-Smirnov test. Subsequently, the ANOVA test was used to evaluate the differences in the amounts (mean counts X 105) of each bacterial specie and to analyze the proportions of each microbial complex. The Paired t-test was used for the intra-oral groups’ analysis (before and after treatment). Adjustments for multiple comparisons were made to evaluate the 40 bacterial species simultaneously. The unit of analysis was the patient. The significance level was set at 5%. The power of the study was calculated at 80% based on the difference found in the general bacterial count, considered as the primary endpoint, of 2.39 (x105) in test group vs. 0.75 (x105) in control group before treatment, considering the sample size of 20:10 individuals, respectively, a standard deviation of 2.22 (x105), and an α value of 5.
Results
All selected participants (n= 20) completed the 3-month follow-up, eight men (40%) and 12 women, with a mean age of 36 years (8.44). Clinical evaluation is shown in Tables 1 and 2 and revealed a significant decrease of BOP (from 85.45% to 59.22%, p < 0.001) (Table 1), PPD (PM, -0.51 mm, p< 0.001; PS, -1.08, p< 0.001, (Table 2) and PAL (PM, -0.47 mm, p= 0.002; PS, -0.82 mm, p< 0.001, table 2) in periodontitis patients after 3 months. However, VPI was not reduced after therapy (from 40.1% to 29.7%, p= 0.089) (Table 1).
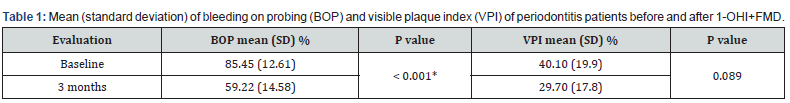
*Statistically significant. Test t. p< 0.05. 1-OHI+FMD: ultrasonic full mouth debridement associated to a single oral hygiene instruction.
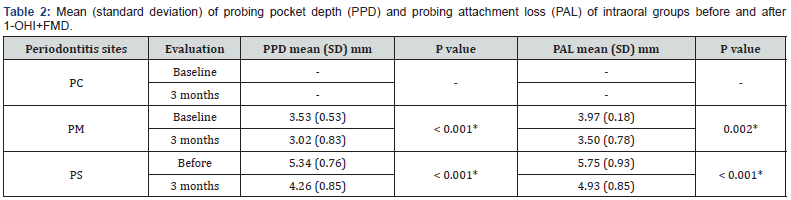
Statistically significant. Test t. p< 0.05. SD: standard deviation. Sites - PC: periodontitis control, saliva; PM: periodontitis, moderate PAL; PS: periodontitis, severe PAL. 1-OHI+FMD: ultrasonic full mouth debridement associated to a single oral hygiene instruction.
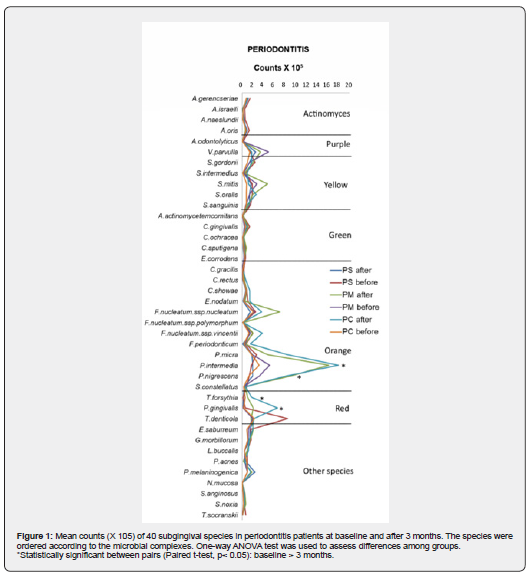
Although no microbiological changes were observed in PC (from 0.85 (±0.94) X 105 to 1.22 (±1.40) X 105, p = 0.326) and PM (from 1.84 (±1.72) X 105 to 1.02 (±0.84) X 105, p = 0.068), 1-OHI+FMD reduced BC in PS from 1.99 (±2.23) X 105 to 0.70 (±0.53) X 105, p = 0.016) after 3 months. Counts of Prevotella intermedia (from 17.94 X 105 to 3.16 X 105, p< 0.001), Prevotella nigrescenses (from 8.2 X 105 to 1.2 X 105, p< 0.001), Tannerella forsythia (from 1.8 X 105 to 0.5 X 105, p< 0.001), and Porphyromonas gingivalis (from 6.5 X 105 to 0.2 X 105, p< 0.001) were only reduced in PS after 3 months (Figure 1). PS also showed a reduction in Orange and Red Complexes (from 60% to 42% and from 13% to 11%, respectively, p < 0.001) but also an increase in Yellow Complex (from 9% to 13%, p < 0.001) (Figure 2).
Discussion
The present study evaluated the clinical and microbiological impact of ultrasonic full mouth debridement associated to a single oral hygiene instruction (1-OHI+FMD) on sites of periodontitis patients with different levels of severity. Periodontitis is a site specific multifactorial inflammatory disease caused by microorganisms [18]. Several studies have demonstrated the relationship between colonization of bacterial plaque-specific microorganisms such as P. gingivalis with the presence and/or severity of periodontitis, [3,19] that increases with age [20]. Twenty patients participated in this study. After baseline examinations, all of them underwent FMD, a fast and uncomplicated therapeutic approach, [8,11-13] associated to a single oral hygiene instruction (1-OHI+FMD). It was observed that there was a significant decrease of PPD, PAL and BOP in all patients, proving the capacity of this therapy to improve clinical parameters [8,12]. Also, microbial analysis revealed a significant counts reduction of some putative pathogens, indicating the microbiological improvement of 1-OHI+FMD approach.
Indeed, to treat periodontitis, the amount of bacteria present should be reduced to allow healing of inflamed tissues [21]. However, the individual bacterial analysis only showed differences in severe sites. This result was partly expected since a former study showed a statistical reduction of periodontopathogens especially in subgingival pockets [22]. It was also observed that counts of P. intermedia, P. nigrescenses, T. forsythia, and P. gingivalis were reduced in PS after 1-OHI+FMD. These results have also been shown before for some putative bacteria, [23] including P. intermedia [24]. The proportion analysis of the bacterial complexes followed these findings and showed reductions in deep sites of periodontitis patients only in red and orange ones.
Although the beneficial effects of 1-OHI+FMD on microbial reduction in patients with periodontitis are undeniable, the persistence of high levels of plaque in all groups is noteworthy. There is growing evidence that patient behavior influences or impairs the success of periodontal treatment [25]. Improved plaque control can be reflected in decreased measurements for plaque accumulation and gingival inflammation [26]. Several studies have shown that FMD is able to reduce the periodontal pocket, promotes attachment gain, decreases BOP, and reduces subgingival microflora [12]. Additionally, it reduces the number of visits, the time in the chair and can be easily applied by most of general dentists [14]. On the other hand, conventional quadrantwise treatment enables a weekly supragingival plaque removal which diminishes counts of supra- and subgingival species, thereby creating a microbial profile comparable to that observed in healthy periodontium [14].
In classical FMD Leuven studies, standard oral hygiene instructions were performed only after the first session of scaling and root planning [14]. As FMD patients did not have the opportunity of receiving repeated oral hygiene instructions and supragingival scaling and tooth cleaning, they may have more difficulty in maintaining a low plaque score. The truth is that is not easy to prevent and treat periodontitis. The high prevalence of periodontal disease proves the difficulty of controlling it [27]. It is important to change patients’ behavior and ensure that they can perform optimal plaque removal [28]. The effectiveness of oral hygiene should be routinely checked to ensure low levels of plaque [27,28]. There are many strategies to instruct, plan, and support behavior change, [28] but all of them take time. Thus, although 1-OHI+FMD is effective in reducing bacterial amount, the short contact time with patients does not seem to offer an opportunity for them to change their behavior and practice proper oral hygiene. A periodontal treatment plan should include repeated oral hygiene instructions and supragingival tooth cleanings until patient has a low plaque index [21]. Futhermore, oral hygiene instructions should be regularly reinforced to maintain the low plaque score [21].
Finally, it is important to highlight some limitations of this study. The split-mouth study design was chosen because it removes a large portion of the inter-individuals variation in the estimates of the treatment effect [29]. However, important issues for clinical trials such as blindness are not feasible to achieve. The sample size calculation for the present study showed an adequate size, but the population of other studies is usually higher. Finally, the 3-month follow-up period could be longer. Several studies follow patients for 6 or even 12 months.
Conclusion
Single oral hygiene instruction associated to ultrasonic full mouth debridement promote a significant improvement in some clinical and microbiological parameters of periodontitis’ patients, especially in deep sites, but it does not seem to be effective in improving the patient’s plaque control.
Acknowledgements
The authors thank the financial support of Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ, Financial support number E-26/102.754/2013) and National Foundation for Development of Higher Education (FUNADESP, Financial support number 1700469).
References
- Cekici A, Kant arci A, Has Turk H, Van Dyke TE (2014) Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontal 2000 64(1): 57-80.
- Kane SF (2017) The effects of oral health on systemic health. Gen Dent 65(6): 30-34.
- How KY, Song KP, Chan KG (2016) Prohormones gingival is: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol 7: 53.
- Shaikh HFM, Patil SH, Pangram TS, Rathod KV (2018) Polymicrobial synergy and dysbiosis: An overview. J Indian Soc Periodontal 22(2): 101-106.
- Safranski SS, Haffajee AD, Cucina MA, Smith C, Kent RL (1998) Microbial complexes in subgingival plaque. J Clin Periodontal 25(2): 134-44.
- Bartold PM, Van Dyke TE (2013) Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000 62(1): 203-217.
- Barros CE, Siqueira V, Carvalho D, Canabarro A (2019) Putative periodontal bacteria in clinically healthy and diseased sites of periodontitis patients. Braz J Oral Sci 18: e191417.
- Shaddox LM, Walker CB (2010) Treating chronic periodontitis: current status, challenges, and future directions. Clin Cosmet Investig Dent 2: 79-91.
- Sanz I, Alonso B, Carasol M, Herrera D, Sanz M (2012) Nonsurgical treatment of periodontitis. J Evid Based Dent Pract 12(3 Suppl): 76-86.
- Drisko CL (2014) Periodontal debridement: still the treatment of choice. J Evid Based Dent Pract 14(Suppl): 33-41.
- Canabarro A, Marcantonio É Jr, De-Deus G (2015) Use of the Strength of Recommendation Taxonomy (SORT) to assess full-mouth treatments of chronic periodontitis. J Oral Sci 57(4): 345-53.
- Pabolu CM, Mutthineni RB, Chintala S, Naheeda, Mutthineni N (2013) Evaluation of the effect of one stage versus two stage full mouth disinfection on C-reactive protein and leucocyte count in patients with chronic periodontitis. J Indian Soc Periodontol 17(4): 466-471.
- Ximénez-Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, et al. (2000) The effect of repeated professional supragingival plaque removal on the composition of the supra- and subgingival microbiota. J Clin Periodontol 27(9): 637-647.
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, et al. (2018) Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89(Suppl 1): S173-S182.
- Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, et al. (2018) Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 45(Suppl 20): S68-S77.
- Ribeiro MHB, Ribeiro PC, Retamal-Valdes B, Feres M, Canabarro A (2019) Microbial profile of symptomatic pericoronitis lesions: a cross-sectional study. J Appl Oral Sci 28: e20190266.
- Tonetti MS, Claffey N (2005) European Workshop in Periodontology group C Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 32 Suppl 6: 210-213.
- Moore LV, Moore WE, Cato EP, Smibert RM, Burmeister JA, et al. (1987) Bacteriology of human gingivitis. J Dent Res 66(5): 989-995.
- Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, et al. (2018) Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Periodontol 89(Suppl 1): S140-S158.
- Aimetti M (2014) Nonsurgical periodontal treatment. Int J Esthet Dent 9(2): 251-267.
- Bollen CM, Mongardini C, Papaioannou W, Van Steenberghe D, Quirynen M (1998) The effect of a one-stage full-mouth disinfection on different intra-oral niches. Clinical and microbiological observations. J Clin Periodontol 25(1): 56-66.
- Swierkot K, Nonnenmacher CI, Mutters R, Flores-de-Jacoby L, Mengel R (2009) One-stage full-mouth disinfection versus quadrant and full-mouth root planing. J Clin Periodontol 36(3): 240-249.
- Bollen CM, Vandekerckhove BN, Papaioannou W, Van Eldere J, Quirynen M (1996) Full- versus partial-mouth disinfection in the treatment of periodontal infections. A pilot study: long-term microbiological observations. J Clin Periodontol 23(10): 960-970.
- Pastagia J, Nicoara P, Robertson PB (2006) The effect of patient-centered plaque control and periodontal maintenance therapy on adverse outcomes of periodontitis. J Evid Based Dent Pract 6(1): 25-32.
- Ximénez-Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, et al. (2000) The effect of repeated professional supragingival plaque removal on the composition of the supra- and subgingival microbiota. J Clin Periodontol 27(9): 637-647.
- Tonetti MS, Eickholz P, Loos BG, Papapanou P, van der Velden U, et al. (2015) Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J Clin Periodontol 42(Suppl 16): S5-11.
- Matthews DC (2014) Prevention and treatment of periodontal diseases in primary care. Evid Based Dent 15(3): 68-69.
- Shoukri MM, Colak D, Donner A (2011) Likelihood inference on the relative risk in split-cluster designs. Clin Trials 8(1): 37-47.






























