Efficacy of Lateral Internal Sphincterotomy (Lis) For Chronic Anal Fissures
Jayalal JA*, Jekin J Sharon and Dhayanidhi
Senior Consultant Laparoscopic Surgeon, Annammal Hospital, India
Submission:February 13, 2025;Published:March 12, 2025
*Corresponding author:Jayalal JA, Senior Consultant Laparoscopic Surgeon, Annammal Hospital, Kuzhithurai, Kanyakumari District, Tamil Nadu, India
How to cite this article:Jayalal JA, Jekin J Sharon and D. Efficacy of Lateral Internal Sphincterotomy (Lis) For Chronic Anal Fissures. Adv Res Gastroentero Hepatol, 2025; 21(4): 556067.DOI: 10.19080/ARGH.2025.21.556067.
Abstract
An anal fissure is a longitudinal rupture in the anoderm below the dentate line. It is one of the most common benign conditions in the anorectal area. Anal fissures can reduce a person’s quality of life because of the severe discomfort they endure during defecation and the emotional stress that follows. There are several treatment options, including medications and surgery. This study looked back at the past to see if lateral internal sphincterotomy (LIS) was a safe and effective way to treat anal fissures that would not go away. The findings suggest that LIS can provide significant relief for patients suffering from chronic anal fissures, leading to improved quality of life and reduced pain during bowel movements. Additionally, the study highlights the importance of considering this surgical option for those who have not responded to conservative treatments.
Method: This retrospective analysis included 200 patients who received treatment for a persistent anal fissure. The patients were assessed for pain levels, healing rates, and overall satisfaction post-surgery. Results indicated that a majority experienced substantial improvements within weeks, reinforcing the procedure’s role as a viable solution for individuals facing enduring discomfort.
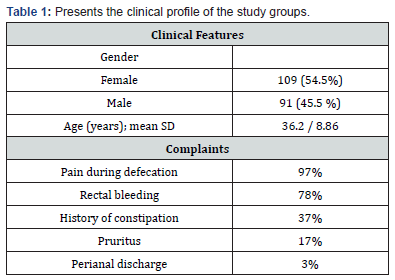
Findings: The mean SD age of the 200 patients in the study was 36.2 ± 8.86 years, with 109 (54.5%) of them being female (ranging from 17 to 73 years). Pain, bleeding, constipation, pruritus, and perianal discharge were among the main symptoms of the patients. Four patients (2%) developed incontinence, including two cases of gas incontinence, two instances of soiling, three female patients, and one male patient. Seven males and one female experienced recurrence. While all patients with gas incontinence had a regression in their problems, three patients developed permanent fluid incontinence.
Conclusion: LIS is still the recommended treatment for chronic anal fissures when doctors want to avoid recurrence and provide the best affordable treatment. The findings indicate that while most patients experience improvement, a small percentage may face ongoing challenges with incontinence. Therefore, it is crucial for healthcare providers to thoroughly discuss potential outcomes and risks with patients before the procedure, ensuring informed consent and setting realistic expectations for recovery.
Keywords:Lateral sphincterotomy; Anal fissure; Incontinence; Pain
Abbreviations:LIS: lateral Internal Sphincterotomy; ASCRS: American Society of Colon and Rectal Surgeons; IBD: Inflammatory Bowel Disease
Introduction
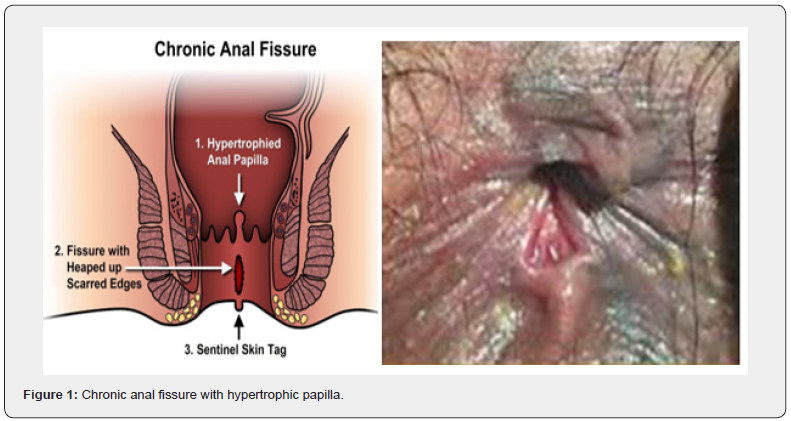
Anal fissures are longitudinal tears in the anoderm that happen below the dentate line. 90% of them are located in the midline of the body, behind the spine [1,2]. It is among the most prevalent benign anorectal disorders. The primary symptoms are rectal bleeding, pain during bowel movements, and emotional stress that can lower a person’s quality of life [3]. The chronic anal fissure has a hypertrophic papilla and a sentinel tubercle. The sphincter muscle fibers at the base of the tear are visible, and it takes longer than 8 to 12 weeks to heal [4,5]. Healing often requires a combination of conservative measures, such as dietary changes and topical treatments, as well as potential surgical interventions in more severe cases. Prompt diagnosis and effective management are essential to alleviate symptoms and restore the patient’s overall well-being.
Physical or pharmacological techniques generally lower the sphincter pressure to treat anal fissures. There is no universally accepted treatment for chronic anal fissures, research on treatment options ranges from surgery to medication applications [6,7]. According to the American Society of Colon and Rectal Surgeons (ASCRS) guidelines, the first line of treatment should be nonsurgical and include warm sitz baths, high-fiber diets, and stool softeners [8]. More treatment alternatives will be needed since many patients will not respond to conservative care. Lateral internal sphincterotomy (LIS) is the best surgery for chronic anal fissures when conservative and drug-based treatments do not work [9,10].
In addition to its effectiveness, LIS carries some risk of complications. While incontinence-the most common and feared complication-was temporary in most cases, 3% had it permanently at 72 months [11]. Our goal in this study was to find the answers to the following five questions:
a) Is it an adequate and safe alternative to open lateral internal sphincterotomy (LIS)?
b) Which LIS early and late problems are more prevalent?
c) What variables influence incontinence and recurrence in patients who have LIS?
d) How can we prevent incontinence?
e) After LIS, is patient satisfaction truly high?
Methodology
Out of the 253 patients who had surgery to repair an anal fissure in the Department of General Surgery in the period from January 2020 to December 2023, 200 individuals were included in the study because of the regular follow-up carried out for a year. Every patient had previously received medical care that included a warm sitz bath, laxative, high-fibre diet, and stool softener. Patients with hemorrhoidal disorders, anal abscesses, anal fistulas, inflammatory bowel disease (IBD), hemorrhoidal disorders, anal abscesses, anal fistulas, or whose medical records were not available were not included in the study. Neither rectal nor anal cancer was a history for the individuals. We gave the patients a questionnaire to complete about their symptoms. A linear visual analog pain score was used to measure anal pain both before therapy and during follow-up appointments. As previously reported by Pescatori et al. [12], anal incontinence was evaluated using a validated score and grading method.
Ethical Approval
The institutional ethics committee approval was obtained, and every patient provided written informed consent. The Declaration of Helsinki’s guiding principles were followed when conducting the study. The study aimed to ensure that all procedures adhered to these ethical standards, thereby safeguarding the rights and well-being of the participants. The data were also looked at to see how well the therapeutic interventions worked and if there were any links between the patients’ reported outcomes and the relief of their symptoms.
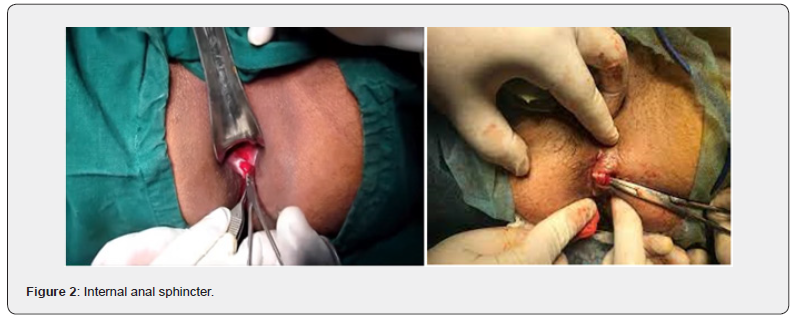
Surgical procedures (Figure 1 & 2)
The surgical procedure, open lateral sphincterotomy, was done in the lithotomy posture under either general or regional anaesthesia. Patients were optimally prepared. No regular enema is used routinely. The anal canal was visualized with an anoscope. At three o’clock, a longitudinal incision of roughly one centimeter was made in the inter sphincter groove, and the muscle and mucosa were separated using an artery. At the end of the procedure, a small dressing was put on the area where the distal half of the internal anal sphincter was separated under direct vision and pressed for three minutes. The incision was then closed with a 3-0 Vicryl suture my, anal fissure, incontinence, pain.
Postoperative management and follow-up
Every follow-up clinic visit involved anorectal inspection of the patients, and we tracked the healing of the fissures. We scheduled postoperative follow-up appointments after one week, two weeks, four weeks, and eight weeks, respectively. A visual analog scale that ranged from 0 (no pain) to 10 (worst pain imaginable) was used to measure pain alleviation. We conducted a retrospective analysis of the patient’s medical records. The patient’s age, medical history, symptoms and results, and reaction to treatment (pain reduction and assessment of the fissure, erythema, and inflammation) were among the characteristics that were examined. We then documented and examined the treatment’s adverse effects and the recurrence rates of the illness.
Results
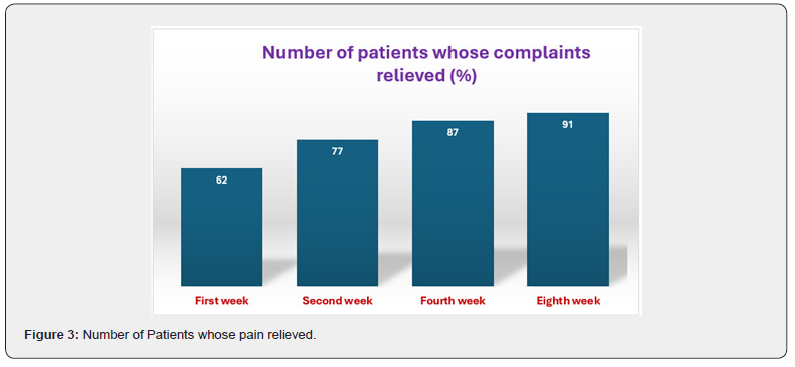
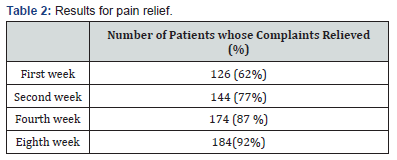
Demographic and clinical profiles of the patients are collected and tabulated in Table 1. Of 200 patients included in the study, 109 (54.5%) were female, and the mean SD age was 36.2 ± 8.86 years (ranging from 17 to 73 years). In the vast majority of the patients, the primary complaints were pain (97%) and rectal bleeding (78%) during and/or after defecation. Perianal discharge, pruritus, and constipation were the other main complaints. The patients were assessed to determine the status of complaints and the response to treatment at the first, second, fourth, and eighth weeks after the start of treatment (Table 2) 126 patients (63%) experienced pain alleviation in the first week, followed by 154 (77%) in the second, 174 (87%) in the fourth, and 182 (91%) in the eighth. At the end of the eighth week, 36 patients showed no signs of relief. The disease lasted a median of 27 months, with a range of 1–34 months. Table 3 displays the procedure’s results with a median follow-up of one year. The distribution of rates of early and late problems per year did not decline (Figure 3). 34 patients (34%) experienced rectal bleeding as a common issue in the early postoperative period.
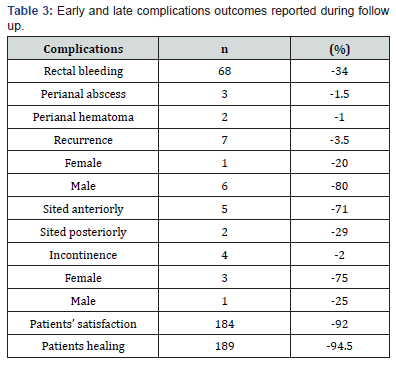
On the process of the treatment, two individuals had the history of using anticoagulants (such as acetylsalicylic acid or clopidogrel) developed perianal hematomas, while three patients presented with perianal abscesses. These individuals saw improvement following the evacuation of the hematoma and abscess. In the long-term follow-up, 4 patients (2%) suffered incontinence (two with gas and two with soiling; 3 females and one male), while 7 patients (3.5%) developed recurrence (5 males and two females). The recurrence rate for anterior fissures was 67%. Two patients had undergone two prior surgeries, while nine patients who experienced recurrence had one. The individual with incontinence had already undergone anorectal surgery, and all the women had already experienced vaginal childbirth. Three female patients experienced persistent fluid incontinence. however, the complaints of all patients with gas incontinence and one male patient with fluid incontinence diminished by the fourth postoperative month.
Additionally, the other major complaints were constipation, pruritus, and perianal discharge. The patients had been evaluated at the first, second, fourth, and eighth weeks from the beginning of the treatment for assessing the response to the treatment and the status of complaints (Table 2). In the first week, 126 patients (62%) experienced pain relief, followed by 144 (77%) in the second week, 174 (87%) in the fourth week, and 182 (91%) in the eighth week. 18 patients did not experience relief by the end of the eighth week
The median duration of the disease was 27 months (ranging from 1 to 34 months). Table 3 shows the outcomes of the procedure with a median follow-up of one year. The results indicate a significant improvement in patient quality of life, with notable reductions in symptom severity. Furthermore, the data suggests that early intervention may correlate with more favorable long-term outcomes. Patient satisfaction was high (92%) and the healing was nearly complete (94.5%) at the end of the eighth week [13].
Discussion
The objectives of our research were to determine the surgical complications associated with lateral internal sphincterotomy (LIS), assess its efficacy in treating chronic anal fissures, and pinpoint the variables that affect these issues. Regarding incontinence and recurrence rates, pain alleviation, patient satisfaction, and healing, our data corroborate previous research in the literature. Subcutaneous lateral internal sphincterotomy was first proposed by Notaras in 1971 [14]. Sphincterotomy’s primary objective is to reduce the maximum anal sphincter pressure by 18 to 50% to enhance the anoderm’s blood flow. This method offers an improvement of 82% to 100%. Araujo et al. [15] conducted a prospective clinical experiment with 190 patients in three groups, comparing LIS (n = 62) and medical treatment (n = 128) and reported that LIS had 100% pain alleviation rates after the eighth week, with 93% of patients experiencing pain reduction after two weeks and 100% at the end of the eighth week.
Vaithianathan et al. [16] assessed preoperative and postoperative pain alleviation using the visual analogue scale (VAS) at the first, fourth, and sixth weeks in a series of 45 patients who had LIS. By the conclusion of the sixth week, nearly all patients had pain relief. Even though our study’s rate was marginally lower (91%) than the other two, we found that most of our cases showed improvement after eight weeks. In the early phases of treatment after LIS, most patients reported feeling satisfied. Just 7% of patients in the LIS group reported being unhappy with their therapy, according to a study comparing the outcomes of Botox and LIS. One In a single-centre investigation, Salih et al. found this rate to be 1% [17]. Garcia-Aguilar et al. [18] reported 80% of patients were either extremely satisfied or satisfied following surgery. Gupta [19] evaluated the impact of excising fibrous anal polyps and hypertrophied anal papillae on patient satisfaction and revealed that patients who had pruritus or dampness around the anal border, pricking or a sensation of a foreign body in the anus, and pain and irritation during bowel movements had a noticeably greater rate of satisfaction.
In our study, over 8% of patients were unhappy with the results of their LIS surgery because of recurrence, incontinence, and/or other early problems. Since the most frequent complaint from patients was the presence of palpable hypertrophic papillae following surgery, we think that removing the fibrous anal polyps and hypertrophied anal papillae will improve patient satisfaction even though there is insufficient evidence to assess this condition. The most notable drawback of LIS is fecal incontinence [20]. It is estimated following LIS, up to 47.6% of patients experienced post-operative disruption of continence in varied degrees of severity [21]. Additionally, there are other studies in the literature that show partial sphincterotomy also lowers the risk of incontinence [22-24]. Following LIS surgery, incontinence usually appears as flatus incontinence or light soiling and lasts for a few weeks to six months. But according to other writers, fecal incontinence might last up to a year [25]. With a clear fecal incontinence rate of 1%, a new meta-analysis found a substantial (about 15%) long-term risk of incontinence after LIS [26].
According to Arroyo et al. [27], recovery rates for patients undergoing LIS operation were 92.5%, and the treatment resulted in anal incontinence in 5% of individuals. Nyam et al. reported that 96% of patients recovered after LIS, and 45% of patients experienced some degree of incontinence. Mentes [11] and colleagues found that incontinence reduced the quality of life for just 1.2% of the 244 patients who had LIS for IAS. Numerous factors, including age, gender-specific anatomical characteristics, history of pelvic surgery, vaginal delivery and pregnancy, surgical inexperience, and incorrect technique, appear to influence the possible risk of FI following LIS. Complications include wound infection, hematoma, abscess, and anal fistula can also occur after LIS [26,28,29]. Our incontinence and recurrence outcomes are based on complete LIS because we did not include those that had partial LIS. 4 patients, or 2% of LIS operations, experienced gas and/or liquid incontinence, while 3 patients who had gas incontinence went away on its own after eight months of no treatment. The remaining one however, continued to have liquid incontinence. Every patient with a history of vaginal delivery who had permanent incontinence was female.
Rectal bleeding was the most common early-stage result, with two patients developing perianal abscess and two patients experiencing perianal hematoma. Hemostasis and abscesses required drainage, but rectal bleeding did not require any additional treatment. Even though fissure healing after sphincterotomy has a high success rate, recurrence might occur in 1.6% to 6% of instances [30]. The most common reason for recurrence is inadequate sphincterotomy. Recurrence is affected by the fissure’s location. In anterior fissures, the recurrence rate is higher [31,32] . We can do a repeat sphincterotomy in these situations. According to our data, the LIS group’s recurrence rate (4%) was consistent with previous research, and young men with anterior fissures experienced the majority of recurrent cases. This is explained by men’s strong pelvic muscles, which result in insufficient sphincterotomy.
Limitations of the Study
This is a single-centre study, with no randomization, and the use of various surgeons for the procedures and retrospective in nature are among its major flaws. Additionally, because anal manometry was not performed, anal canal pressures were unknown. The lack of a comparative analysis with different treatment approaches is a significant drawback of this study. However, despite being a single-centre experiment, the large patient population is its main advantage. We claim that surgeons doing anorectal surgery will greatly benefit from our objectively communicated LIS results.
Conclusion
This study’s findings indicate elevated healing and patient satisfaction rates following LIS. Given that the most dreaded complication of LIS is irreversible incontinence, meticulous patient selection during the preoperative phase is essential, sphincter pressure must be assessed, and other therapies such as Botox should be contemplated for patients at elevated risk of incontinence. Furthermore, the surgeon must ensure that the sphincterotomy is executed correctly to minimize recurrence rates.
References
- Brady JT, Althans AR, Neupane R (2017) Treatment for anal fissure: is there a safe option? Am J Surg 214: 623-628.
- Collins EE, Lund JN (2007) A review of chronic anal fissure management. Tech Colorectal 11: 209-223.
- Gil J, Lujan J, Hernandez Q, Gil E, Salom MG (2010) Screening for the effectiveness of conservative treatment in chronic anal fissure patients using anorectal manometry. Int J Colorectal Dis 25: 649-654.
- Wald A, Bharucha AE, Cosman BC, Whitehead WE (2014) ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol 109: 1141-1157.
- Andjelkov K, Sforza M, Barisic G, Soldatovic I, Hiranyakas A (2017) Novel method for treatment of chronic anal fissure: adipose-derived regenerative cells e a pilot study. Colorectal Dis19: 570-575.
- Acheson AG, Scholefield JH (2005) Anal fissure: the changing management of a surgical condition. Langenbeck’s Arch Surg 390: 1-7.
- Tayfun Y, Dogan G, Mahmut O, Ferda NK, Sibel G (2009) Comparison of controlled-intermittent anal dilatation and lateral internal sphincterotomy in the treatment of chronic anal fissures: a prospective, randomized study. Int J Surg 7: 228-231.
- Perry WB, Dykes SL, Buie WD, Rafferty JF (2010) Standards practice task force of the American Society of Colon and Rectal Sur- geons Practice parameters for the management of anal fissures (3rd. revision). Dis Colon Rectum 53: 1110-1115.
- Nelson RL, Chattopadhyay A, Brooks W, Platt I, Paavana T (2011) Operative procedures for fissure in ano. Cochrane Database Syst Rev 9: CD002199.
- Hsu TC, MacKeigan JM (1984) Surgical treatment of chronic anal fissure. A retrospective study of 1753 cases. Dis Colon Rectum 27: 475e478.
- Mentes BB, Tezcaner T, Yılmaz U, Leventoglu S, Oguz M (2006) Results of lateral internal sphincterotomy for chronic anal fissure with particular reference to quality of life. Dis Colon Rectum 49: 1045-1051.
- Pescatori M, Anastasio G, Bottini C, Mentasti A (1992) New grading and scoring for anal incontinence. Evaluation of 335 patients. Dis Colon Rectum 35: 482-487.
- Jayalal JA (2023) Prophylactic Antibiotics Compliance and its Impact on SSI -A Prospective Study. Int J Acad Med Pharm 5(3): 884-888.
- Notaras MJ (1971) The treatment of anal fissure by lateral subcutaneous internal sphincterotomy: a technique and results. Br J Surg 58: 96-100.
- Poh A, Tan KY, Seow-Choen F (2010) Innovations in chronic anal fissure treatment: a systematic review. World J Gastrointest Surg 2: 231-241.
- Araujo SE, Sousa MM, Caravatto PP, Habr-Gamai A, Cecconello I (2010) Early and late results of topical diltiazem and bethanechol for chronic anal fissure: a comparative study. Hepato-Gastroenterology 57: 81-85.
- Vaithianathan R, Panneerselvam S (2015) Randomised prospective controlled trial of topical 2% diltiazem versus lateral internal sphincterotomy for the treatment of chronic fissure in ano. Indian J Surg 77: 1484-1487.
- Salih AM (2017) Chronic anal fissures: open lateral internal sphincterotomy result: a case series study. Ann Med Surg (Lond) 15: 56-58.
- Garcıa-Granero E, Sanahuja A, Garcıa-Botello SA (2009) The ideal lateral internal sphincterotomy: clinical and endosono- graphic evaluation following open and closed internal anal sphincterotomy. Colorectal Dis 11: 502-507.
- Gupta PJ (2004) Hypertrophied anal papillae and fibrous anal polyps, should they be removed during anal fissure surgery? World J Gastroenterol 10: 2412-2414.
- Khan MS, Akbar I, Zeb J, Ahmad S, Khan A (2017) Outcome of 0.2% glyceryl trinitrate cream versus 2% diltiazem cream in the treatment of chronic anal fissure. J Ayub Med Coll Abbottabad 29: 280-284.
- Chen HL, Woo XB, Wang HS (2014) Botulinum toxin injection versus lateral internal sphincterotomy for chronic anal fissure: a meta-analysis of randomized control trials. Tech Coloproctol 8: 693-698.
- Nessar G, Topbas M (2017) Lateral internal partial sphincterotomy technique for chronic anal fissure. Indian J Surg 79: 185-187.
- Emile SH, Youssef M, Elfeki H, Thabet W, El-Hamed TM (2016) Literature review of the role of lateral internal sphincterotomy (LIS) when combined with excisional haemorrhoidectomy. Int J Colorectal Dis 31: 1261-1272.
- Nyam DC, Pemberton JH (1999) Long-term results of lateral internal sphincterotomy for chronic anal fissure with particular reference to incidence of fecal incontinence. Dis Colon Rectum 42: 1306-1310.
- Jayalal JA (2024) Efficacy of Ligation 0f Inter sphincteric Fistulous Tract (LIFT) In Cryptoglandular Abscess. International Journal of Science and Research (IJSR) 13(7).
- Garg P, Garg M, Menon GR (2013) Long term continence disturbance after lateral internal sphincterotomy for chronic anal fissure: a systematic review and meta-analysis. Colorectal Dis 15: 104- 117.
- Arroyo A, Perez F, Serrano P, Candela F, Lacueva J (2005) Surgical versus chemical (botulinum toxin) sphincterotomy for chronic anal fissure: long-term results of a prospective randomized clinical and manometric study. Am J Surg 189: 429-434.
- Poritz LS. Anal fissure treatment and management. Medscape.
- Whatley JZ, Tang SJ, Glover PH (2015) Management of complicated chronic anal fissures with high-dose circumferential chemo denervation (HDCC) of the internal anal sphincter. Int J Surg 4: 24-26.
- Lindsey I, Cunningham C, Jones OM, Francis C, Mortensen NJ (2004) Fissurectomy-botulinum toxin: a novel sphincter-sparing pro- cedure for medically resistant chronic anal fissure. Dis Colon Rectum 47: 1947-1952.
- Emile SH (2017) Indications and technical aspects of internal anal sphincterotomy: highlighting the controversies. Dis Colon Rectum 60: 128-132.






























