Abstract
Extreme temperatures and water deficits pose a significant threat to crop growth and food security in changing climates. Maize, a widely distributed crop, is susceptible to water deficiency. Seed priming, a low-cost and sustainable technology, can enhance drought tolerance, potentially improving crop productivity and food security. The current study aimed to investigate the impacts of seed thermopriming at physiological and biochemical levels under drought stress during the reproductive stage using three maize genotypes (Red, White, and P-3057w). The experiment was split into six treatments (control, drought, thermopriming at 40 °C, thermopriming at 40 °C + drought, thermopriming at 50 °C, and thermopriming at 50 °C + drought). Drought stress was induced at the pre-tasseling stage by withholding irrigation for 20 days in a split-plot under a completely randomized design. Our results showed that the drought stress significantly reduced photosynthetic rate (51%), stomatal conductance (65%), transpiration (69%), cell membrane injury (55%) chlorophyll a (85%), chlorophyll b (74%), total chlorophyll (81%) and carotenoids (66%) while increasing anthocyanins (7%), phenols (8%), DPPH (6%) and ABTS (11%) activity in non-primed seeds whereas thermopriming at 40oC and 50oC reduced significantly the negative effects of drought on photosynthesis (18%; 21%), stomatal conductance (16%), transpiration (15%; 16%) and cell membrane injury (36%; 37%) through increased anthocyanin content (25%; 24%), total phenols (30%; 29%), DPPH (10%; 11%) and ABTS activity (16%; 17%). However, there were no significant differences between thermopriming at 40 °C and 50 °C. Consequently, both thermopriming treatments were found to be effective for increasing the drought stress tolerance during the reproductive stage in maize.
Keywords: Anthocyanins; Cross stress tolerance; Photosynthesis; Reproductive stage
Abbreviations: Pn: photosynthetic rate
Introduction
The world’s population is expected to increase significantly by 2050. As a result, it is believed that there will not be enough land available to cultivate cereal crops and meet the growing global demand for food. The loss of agricultural land as a result of climate change, the increase in biotic and abiotic stressors, and the acceleration of global climate change are some of the primary challenges facing agriculture and food production [1]. Drought, heat, salinity, and cold are examples of environmental stressors that can hinder crop quality and yield. Global climate change is predicted to increase the frequency and severity of severe weather events, such as extreme temperatures and decreased precipitation [2]. In the final decade of the 20th century, the average global temperature increased by 0.3 °C, and by 2100, it is expected to have increased by nearly 3 °C [3]. Two of the most prevalent abiotic stressors that can impact crop growth and productivity, and ultimately food se curity in a changing climate, are extreme heat and water scarcity [4]. Additionally, they have the potential to significantly alter the composition of the germplasm [5,6].
Maize (Zea mays L.), the most widely distributed crop in the world, is grown in temperate, tropical, and subtropical regions [7]. It ranks as the third most significant crop globally after rice and wheat, with a production of 1241.56 million metric tons over 208.23 million hectares of cultivated land [8]. Maize is more susceptible to drought than crops like sorghum, resulting in a 66% decrease in maize yield and a 33% decrease in sorghum yield [9].
Drought stress is known to be the most harmful abiotic stressor to crops, affecting the growth, development, and production of a wide range of crops. It changes the physiology, biochemistry, and morphology of a plant [10]. Plant photosynthesis is severely hampered by water deprivation stress because it greatly reduces the concentrations of vital photosynthetic pigments in the majority of plants, which slows down the rate at which CO2 is absorbed [11]. Plants have evolved several defensive mechanisms, such as an increase in antioxidant compounds, to fend off oxidative damage from drought. Polyphenolic compounds are essential for mitigating the negative impact of stress [12]. The phenolics production in plants is a complicated web of reactions that are either endogenously controlled or triggered by external stimuli like such as moisture [13]. In reaction to environmental stresses, phenols accumulate to protect tissues from oxidative stress by free radicals [14]. The reproductive period of maize plants, which occurs between tassel development and silking, is the most susceptible to water scarcity. Gas exchange, leaf water content, photosynthetic activity, chlorophyll a and b content, and cell membrane flexibility are all negatively impacted by this stage [15,16].
Given the world’s expanding population, new effective plant breeding methods are needed to improve crop quality, boost yield output, raise stress tolerance, and develop more sustainable and adaptable germplasm pools for future climatic issues [17]. Numerous studies have been conducted on the intricate networks of stress response and adaptation mechanisms of high-value crops, like soybean (Glycine max), wheat (Triticum aestivum), rice (Oryza sativa), and maize (Zea mays) [18-21]. One of the recommended management techniques to treat drought stress is stress priming, often referred to as stress hardening, training, or conditioning. It is a low-cost and environmentally friendly technology. Plants can develop either short-term or long-term stress memory, which increases their resistance to stressors in the present or even in subsequent generations [22,23]. Reports of the phenomenon known as “plant stress memory,” which characterizes the enduring effects and stress imprint where past exposures to biotic and/or abiotic stress have significantly influenced future stress responses, have increased in recent years [17]. The capacity of a plant to grow more resistant to a range of abiotic stressors and, in some cases, biotic stressors following exposure to a single stressor is known as cross-stress tolerance [24]. One possible outcome of stress exposure to plants is the establishment of stress memory, which makes them more resistant to future stressors. This process is also referred to as priming, acclimatization, conditioning, or hardening [25]. There is a lag or memory phase during priming between the priming event and the second stress event [26]. During the priming phase, physiological, metabolic, molecular, and epigenetic alterations take place. Throughout a plant’s life, these alterations might be transient or long-lasting, and in some cases, they can even be inherited by subsequent generations [27]. Stress memory depends on the imprints left on chromatin which subsequently effect the transcription of gene upon the initial stress event, or on the regulators that controls the post-transcription expression of the gene, to either stimulate or inhibit the buildup of transcription factors, signaling metabolites, and proteins produced in response to stress through activating or silencing genes [28,29]. These regulators interact to fine-tune their molecular activities in a variety of abiotic stress scenarios, including heat, cold, drought, and floods. Small noncoding RNAs (sRNAs), including small-interfering RNAs (siRNAs) and miRNAs, are commonly responsible for mediating the feedback loop [29,30]. Many types of plants have been seen to exhibit stress cross-tolerance to a wide range of abiotic stressors brought on by heat priming. Thanks to previous studies on cross-stress tolerance, researchers have been able to link various individual stress responses and begin to clarify the molecular and physiological mechanisms involved in signal initiation and transduction that impart stress tolerance [31]. Pre-sowing priming compounds improve seed germination ability under demanding conditions. Thus, by pre-stimulating the plant’s antioxidant defense mechanism, it is possible to create a stress memory and increase stress tolerance [32]. The effects of preconditioning vary according to plant species and concentration [33]. Using the heat priming method, seeds are kept in a dark environment at a high temperature for certain periods. Germination rates are positively impacted by treatments with either hot or cold temperatures before planting [34]. Heat priming has a positive effect on seed germination and seedling emergence while also encouraging plant growth and development. It has also been demonstrated to enhance enzyme performance, plant growth, and metabolism. Uncertainty surrounds the effect of seed thermal priming on maize’s resistance to drought stress throughout maturity. Understanding the physiological and biochemical impacts of seed thermopriming and drought stress on maize was the aim of this investigation.
Materials and Methods
Plant material and site description
This study examined the physicochemical response of maize throughout the reproductive stage to seed thermopriming under drought stress. For this investigation, two native genotypes of maize (white and red) were gathered from farmers in Aramberri, Nuevo León, Mexico, and one hybrid (P-3057w) was gathered from farmers in Miguel Aleman, Tamaulipas, Mexico. From February to April of 2024, the experiment was carried out at the Facultad de Agronomía, Universidad Autonoma de Nuevo León in Marín, Nuevo Leon, Mexico (located at 24°19’16.71”N and 99°54’58.06” W).
Seed thermopriming treatments
Three different heat stress treatments, i.e., control, 40 °C, and 50 °C, were used. Before being sown, seeds of the three genotypes of maize were exposed to high temperatures of 40 °C and 50 °C for 72 hours in an oven.
Drought treatment
Each genotype’s seeds were planted in germination trays, and when the plants had three leaves, they were moved into 42-liter pots filled with a mixture of peat moss, chicken manure, and black soil (1:1:1). Six treatments were used in the experiment: control, drought, heat priming at 40°C, heat priming at 40°C + drought, heat priming at 50°C, and heat priming at 50°C + drought. There was one plant per pot in a split-plot with a randomized design, and each pot had 26 kg of substrate. To avoid drought stress, all of the pots received regular irrigation until stage V7 (pre-heading). All the pots were irrigated to saturation the afternoon before the drought stress initiation, following an overnight drainage. A small hole was made to re-irrigate the pots of the control treatment as frequently as needed. After that, the pots were enclosed around the stem to avoid direct evaporation of soil water. In the drought treatment, the irrigation was withheld for twenty days. After twenty days, when the plants showed the signs of wilting or leaf rolling, particularly in the morning, data collection was made.
Data collection
Measurements of photosynthetic and biochemical parameters were taken when the plants attained the reproductive phase (VT).
Photosynthetic parameters
The LiCor-6400 (LI-COR Inc., Lincoln, NE, USA) with a 6400- 02B LED light source was used to quantify the photosynthesis variables using the flag leaf, including the net photosynthetic rate (Pn, μmol CO2 m-2 s-1), stomatal conductance (gs, mol H2 O m-2 s-1), and transpiration rate (Tr, mmol H2 O m-2 s-1). During the measurements, the following calibration conditions were used: 700 μmol s-1 of flow, 400 μmol CO2 at constant levels, and a 6400-02 LED light source at 1500 μmol m-2 s-1 [35].
Cell membrane damage
The protocol of Tas [36] was used to quantify cell membrane damage. A 100 mg sample was taken from each randomly chosen leaf, weighed, and then rinsed three times with distilled water. After that, the plant samples were put into test tubes with 10 mL of deionized water. After being sealed, the tubes were placed in a water bath at 32°C for two hours. To find the initial electrical conductivity (EC1) value, the solution’s electrical conductivity (EC) was measured with an electrical conductivity meter. To eliminate all cells and allow organic and inorganic ions to enter the solution, the samples were autoclaved for 20 minutes at 120°C in the second phase. After the samples were brought to room temperature, the electrical conductivity of the solutions was again measured to determine a second electrical conductivity (EC2) value. The cell membrane damage (CMD, %) value was calculated using the following equation:

Photosynthetic pigments
Carotenoids and chlorophyll were measured using the methodology of Rodriguez-Salinas et al. [37]. 10 mL of 80% acetone was added to 100 mg of leaf material in test tubes, which were then agitated for half an hour at room temperature. Following the solution’s filtration, absorbance was measured at 663, 645, 480, and 510 nm and reported in mg g-1 of fresh material. Using the following formulae, the amount of carotenoids and chlorophyll was determined:




Where:
W = Weight of the sample.
V= Volume of the solution.
Total Anthocyanins
The methodology described by Rodriguez-Salinas et al. [37] was used to extract total anthocyanins. 5 mL of an acidified ethanol solution (ethanol and 1 N HCl, 85:15 v/v) adjusted to pH = 1 was added to 200 mg of leaf sample that had been weighed in a test tube. The mixture was then purged with nitrogen flow for 30 seconds. After that, it was kept out of the light at 4 °C for 30 minutes at 200 rpm on a stirrer plate. The supernatant was then extracted and examined at a wavelength of 535 nm after it had been centrifuged for 20 minutes at 4 °C at 6000 rpm. A sample’s anthocyanin concentration was expressed as milligrams of cyanidin- 3 glucoside equivalent (C3GE) per one hundred grams of sample (mgC3GE 100g-1) as follows:

Where:
C = anthocyanin concentration (mgC3GE L-1).
A = sample absorbance.
Ɛ = molar extinction coefficient of cyanidin-3-glucoside
(25.965 cm-1 M-1).
V = total volume of the extract.
MW = molecular weight of cyanidin-3-glucoside (449 g mol).
Total phenols and antioxidant activities
Using the protocol of Rodriguez-Salinas et al. [37], a colorimetric method based on the Folin-Ciocalteau reagent reaction was used to quantify the total phenolic content. 2 mL of phenolic extract were mixed with 2.6 mL of distilled water, oxidized with 0.2 mL of Folin-Ciocalteau reagent, and neutralized with 2 mL of a 7% Na2 CO3 solution after five minutes. After 90 minutes, the reaction was stopped, and the samples’ absorbance at 750 nm was finally measured. Results were reported as milligrams of gallic acid equivalent per hundred grams of sample, with gallic acid serving as a reference for the calibration curve (0, 40, 80, 120, 160, and 200 mg L-1) (mgGAE 100g-1).
Antioxidant activity was assessed using DPPH and ABTS, following the methodology of Rodriguez-Salinas et al. [37]. A 60 μM working solution with an absorbance set to 1.0 at 517 nm was used to assess DPPH. To perform the test, 0.2 mL of phenolic extract and 3 mL of DPPH working solution were combined. The reaction was then allowed to sit in the dark for 30 minutes, and the amount of DDPH that was reduced was measured. 1 mL of 7.4 mM ABTS and 1 mL of 2.6 mM K2S2O8 were combined to create a working solution, which was then allowed to react for 12 hours in the dark. The working solution’s absorbance was then adjusted to 1.0 at 734 nm by diluting it with methanol. To perform the ABTS assay, 0.2 mL of phenolic extract and 3 mL of ABTS working solution were combined. The reaction was then allowed to sit in the dark for two hours, and the amount of ABTS that was reduced was quantified.
Statistical Analysis
This study used a split-plot design with three replications, totaling fifty-four pots, and a randomized design. The normality of the repeated data was evaluated by the Shapiro-Wilk test. Following the normal distribution, the data were then submitted to a two-way analysis of variance (ANOVA) using Statistix 10 software (Analytical Software, FL, USA), where genotypes were assumed as sub-plot component and treatments as main plot factor. The mean comparison was determined using the Tukey test (p≤ 0.05).
Results
As indicated in Table 1, every physiological and biochemical parameter examined for priming treatments under drought stress had significant outcomes (p ≤ 0.05).

Physiological parameters and cell membrane damage
Drought caused a notable decrease in stomatal conductance (65%), transpiration (69%), and photosynthetic rate (51%), as well as a 55% increase in cell membrane damage, in unprimed seeds of all genotypes. With a 58% decrease in photosynthetic rate, a 71% decrease in stomatal conductance, a 78% decrease in transpiration, and a 54% rise in cell membrane damage, the white genotype had the most damage in physiological measures. 51% less photosynthetic rate, 67% less stomatal conductance, 61% less transpiration, and 56% less cell membrane damage were seen in P-3057w, whereas 48% less photosynthetic rate, 57% less stomatal conductance, 66% less transpiration, and 53% less cell membrane damage were observed in the red genotype. The physiological damage caused by drought stress was significantly reduced by heat priming at 40 °C and 50 °C. In plants, 40 °C priming decreased photosynthetic rate, stomatal conductance, transpiration, and cell membrane damage to 18%, 16%, 15%, and 36%, respectively, whereas 50 °C priming decreased drought damage to 21% in photosynthetic rate, 16% in stomatal conductance and transpiration, and 37% in cell membrane injury (Figure 1a-g).
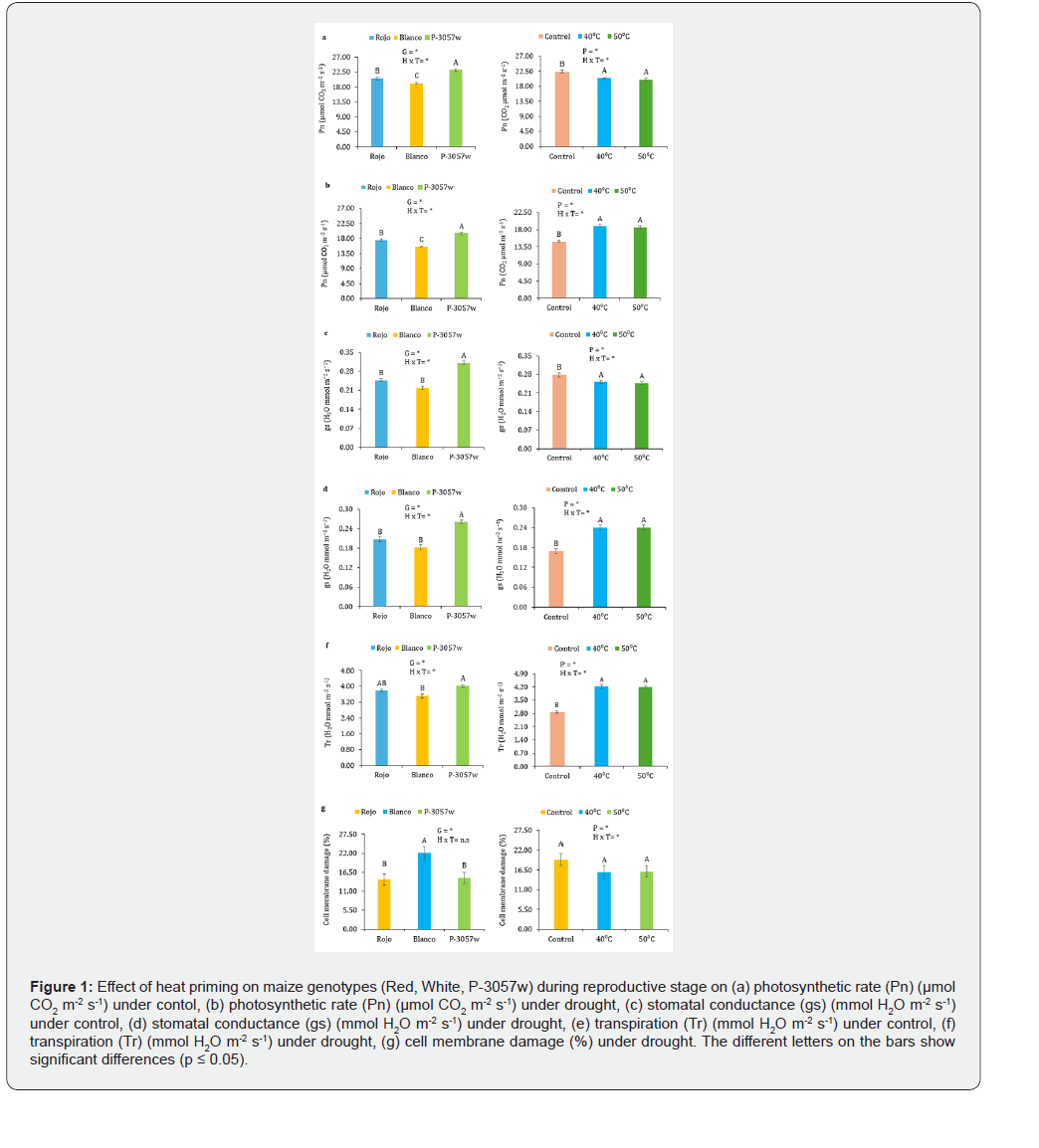
Photosynthetic pigments
Plants cultivated from unprimed seeds under drought stress showed a significant decrease in chlorophyll a (85%), chlorophyll b (74%), total chlorophyll (81%), and carotenoids (66%). The red genotype showed the greatest damage to photosynthetic pigments, with reductions of 90% in chlorophyll a, 103% in chlorophyll b, 95% in total chlorophyll, and 57% in total carotenoids. The white genotype showed reductions of 89% in chlorophyll a, 58% in chlorophyll b, 78% in total chlorophyll, and 86% in total carotenoids, while P-3057w showed reductions of 77% in chlorophyll. Heat priming at 40 °C and 50 °C considerably reduced the physiological harm brought on by drought stress. Chlorophyll a, chlorophyll b, total chlorophyll, and total carotenoids dropped to 20%, 26%, 23%, and 28% in plants primed with 40 °C, whereas drought damage dropped to 19% in chlorophyll a, 14% in chlorophyll b, 17% in total chlorophyll, and 18% in total carotenoids in plants primed with 50 °C (Figure 2a-e).
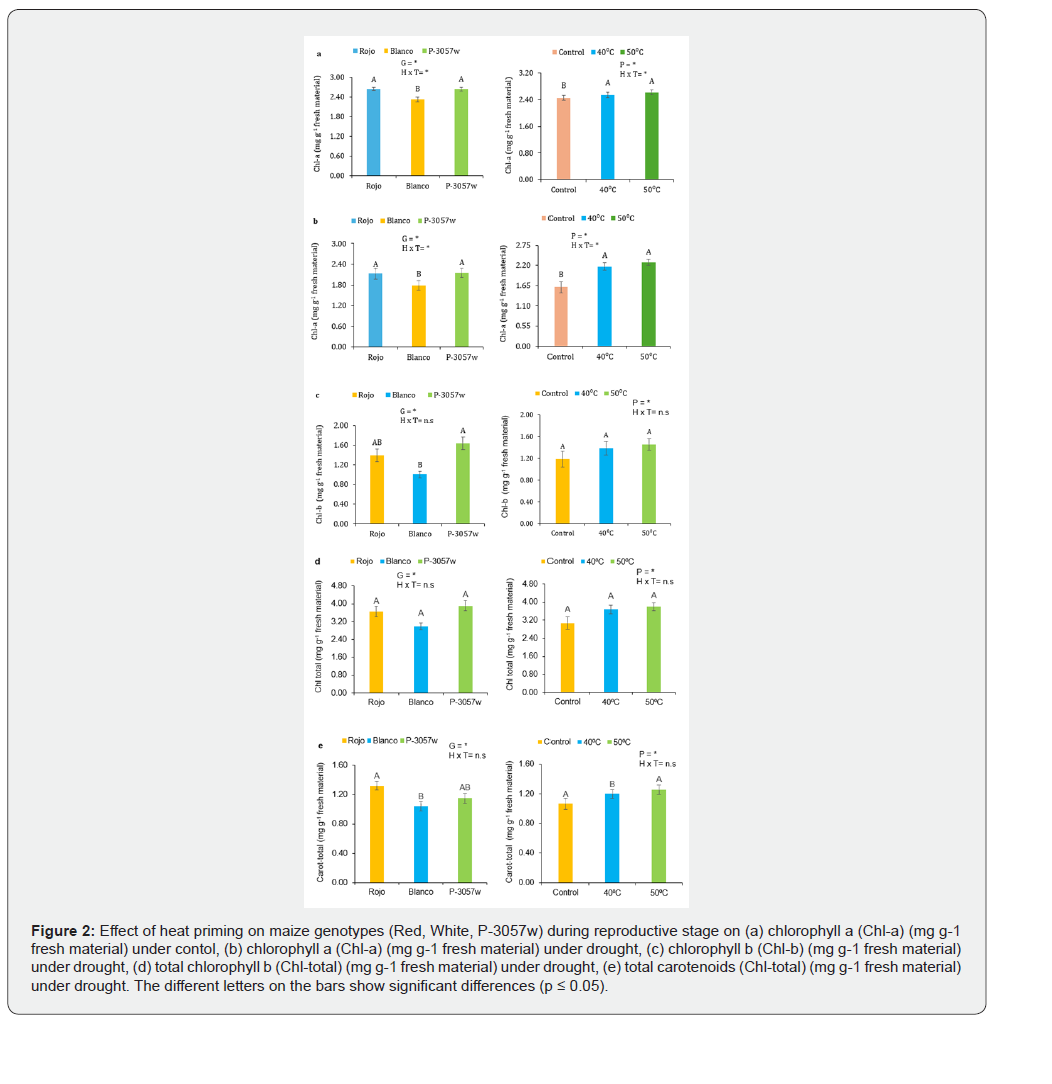
Anthocyanins, Phenols, and Antioxidant Activities
In plants cultivated from unprimed seeds, drought stress raised the levels of anthocyanins (7%), total phenols (8%), and antioxidant activities by DPPH (6%) and ABTS (11%). P-3057w had an 11% increase in anthocyanins, 2% in total phenols, 7% and 11% in DPPH and ABTS activities, and a 1% increase in anthocyanins, 6% in total phenols, 4% and 9% in DPPH and ABTS activities. The red genotype had a greater increase in anthocyanins (8%), total phenols (15%), and antioxidant activities by DPPH (7%) and ABTS (14%). Heat priming, on the other hand, improved the anthocyanins, total phenols, and antioxidant activities, which lessened the damage brought on by drought. Plants primed with anthocyanins at 40 °C showed increases in total phenols, DPPH, and ABTS activity of 25%, 30%, 10%, and 16%, respectively. In contrast, plants primed at 50 °C showed increases in anthocyanin content of 24%, total phenols of 29%, DPPH activity of 11%, and ABS activity of 17%. Heat priming at 40 °C and 50 °C increased anthocyanins (26%, 27%), total phenols (40%, 36%), and antioxidant activities by DPPH (13%, 14%) and ABTS (20%, 21%) the most in pigmented red genotypes (Figure 3a-g).
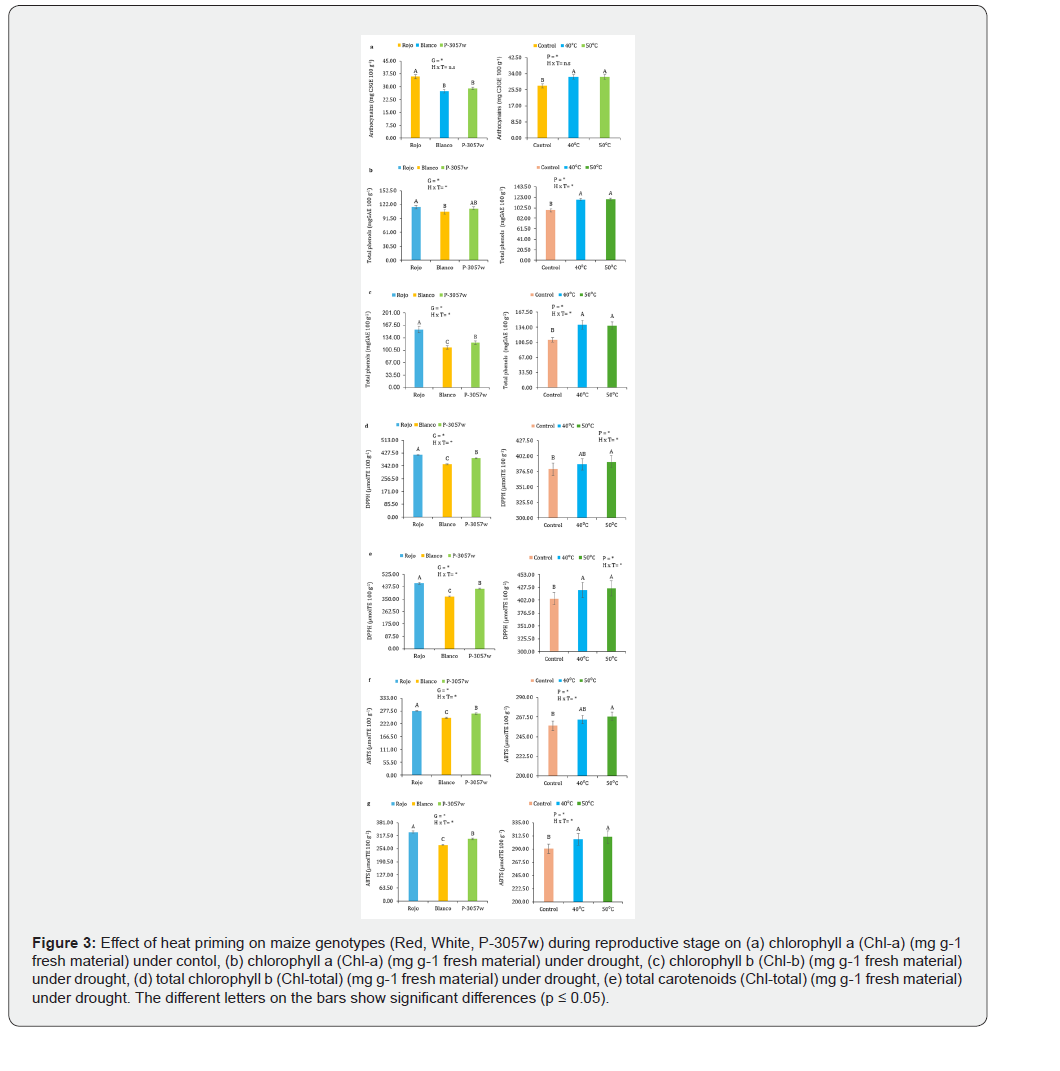
Discussion
Photosynthetic pigments and gas exchange attributes
In a water-limited environment, plants experience reduced photosynthesis, slowed leaf expansion, stomatal narrowing and blockage, early leaf catabolism, decreased translocation acquisition, and decreased crop yield [38-41]. Drought also affects plant-water relations, such as transpiration rate, stomatal opening, leaf water capacity, comparative water accumulation, efficient water utilization, and plant canopy temperature [42,43]. Plants grown under drought have much lower levels of total chlorophyll and carotenoid content than plants cultivated in normal irrigation (Figure 2d,e). Similarly, the water deficit environment significantly impacted several gaseous exchange parameters and stomatal behavior, which significantly decreased when compared to the plants growing in the control treatment (Figure 1a-f). Among other regulatory mechanisms that reduce net photosynthesis, plants that experience drought have decreased stomatal behavior and photosynthetic pigments [44]. We found that plants grown from unprimed seeds under drought stress had reduced rates of photosynthesis, photosynthetic pigments, and stomatal conductance in comparison to plants grown in a control condition. It was previously demonstrated that plants with adequate water supplementation had much higher levels of photosynthetic pigments, gaseous exchange parameters, and stomatal apertures than plants grown in water-limited conditions [39,45,46]. A similar trend was also seen in maize plants under drought stress circumstances [4,15,16].
Additionally, the decline in transpiration rate might be due to the plants’ inability to maintain field water capacity, which was most likely caused by high canopy transpiration [47]. We also saw an electrolyte leak due to cell membrane degradation in unprimed plants under drought stress. Heat-induced seed priming significantly reduced the damage to photosynthetic pigments, gaseous exchange parameters, and cell membrane damage in this study. In this work, seed priming at 40 °C and 50 °C significantly increased photosynthetic rate (28%, 24%), stomatal conductance (42%, 42%), and transpiration (47%, 46%), while the cell membrane damage index during drought significantly decreased by 28%. In plants cultivated from seeds primed at 40 °C and 50 °C, we found a considerable rise in chlorophyll a of 54% and 55%, respectively. Under dryness, there was no discernible change in chlorophyll b, total chlorophyll, or carotenoids between heat-primed and control plants. Similar results were reported by Luqman et al. [48], who discovered that seed priming treatments significantly boosted photosynthetic rate, stomatal conductance, and transpiration in maize hybrids under dry circumstances as compared to the control. According to Zhao et al. [49], drought stress in unprimed wheat seedlings severely compromised cell membrane integrity and increased electrolyte leakage by 33%, whereas priming reduced it by 23%. Ru et al. [50], Hussain et al. [51], and Sen & Puthur [52] have found that seed priming significantly reduced the damage that drought caused to photosynthetic pigments such as carotenoids and chlorophyll. They also found that priming had improved the integrity of the cell membrane by reducing electrolyte leakage during drought stress.
Anthocyanins, Total phenols, and Antioxidant capacities
Abiotic stresses cause plant cells to release reactive oxygen species (ROS). These ROS can lead to cellular dysfunction and death because of their high reactivity with a variety of biological components, including proteins, lipids, nucleic acids, and cell membranes [53]. Plants respond to abiotic stresses by encouraging the production of secondary metabolites that are antioxidants, particularly polyphenolic compounds, as a means of defense and adaptation [54]. Plant resistance to a range of abiotic stresses, including salt, heavy metal toxicity, drought, heat stress, chilling damage, UV radiation, and others, is significantly increased by these chemicals [55,56]. The enhanced production and rapid accumulation of these molecules, which have potent antioxidative properties and may efficiently quench ROS to prevent cellular membrane damage, are essential markers of a plant’s tolerance and resistance to oxidative stress [57]. Research indicates that plants under stress start the synthesis of polyphenolic compounds more quickly than plants developing normally [58-62]. Important enzymes like PAL and CHS are crucial for regulating phenolic synthesis, and complex enzymatic pathways are involved in the regulation of phenolic compound production under stress. Plants control several genes that encode vital enzymes under various abiotic stressors, such as PAL, C4H, C3H, 4CL, COMT, CHS, CHI, F3H, DFR, F3′M, FLS, ANR, and ANS [63].
The enhanced synthesis of a range of bioactive polyphenolic compounds under adverse climatic circumstances results from the increased expression of these genes, which in turn enhances plant resilience through intricate stress tolerance pathways. Plants use several survival strategies, such as the production of secondary metabolites like flavonoids and phenolics, when they are water-limited. The buildup of polyphenolic compounds, such as anthocyanins and other flavonoids, is impacted by drought stress [64]. In this study, heat-induced seed priming dramatically raised the levels of anthocyanins and total phenols. The antioxidant activity in the genotypes under investigation was demonstrated to increase with seed thermopriming at 40 °C and 50 °C. This greatly reduced the physiological damage caused by drought stress and may have improved the genotypes’ ability to tolerate drought. When seeds were thermoprimed at 40 °C and 50 °C, the Red genotype showed the highest increase in antioxidant capacity by DPPH (13%, 14%) and ABTS (20%, 21%), respectively, following P-3057w and White genotypes. Our results are consistent with those of Samota et al. [65], who discovered that plants grown from primed seeds exhibited noticeably greater levels of DPPH antioxidant capacity and total phenols. Hussain et al. [51] reported that seed priming enhanced the total phenolic contents and antioxidant activity in rice genotypes under drought stress; however, the benefits of priming were more pronounced in rice that was pigmented during drought. Another study of the grain by Zhao et al. [49] found that the ABTS and DPPH radical scavenging activities of wheat seedlings increased in response to drought stress. It has been shown that the concentration of phenolic chemicals is the reason for this increase in antioxidant capability.
Conclusion
By boosting antioxidant activity in every genotype examined, seed thermopriming in this study demonstrated a decrease in physiological damage caused by drought stress. Nonetheless, no discernible variations were found between pretreatments conducted at 40 °C and 50 °C. Thus, both treatments were shown to be successful in improving drought tolerance in maize throughout the reproductive stage.
Acknowledgement
This work has been financially supported by CONAHCYT through a scholarship given to the first author, Saba Yasin (CVU: 1193352), for a PhD program at the Facultad de Agronomía, Universidad Autónoma de Nuevo León. The funding source is not involved in the study’s execution or the submission of the publication.
References
- Hossain A, Krupnik TJ, Timsina J, Mahboob MG, Chaki AK, et al. (2020) Agricultural land degradation: processes and problems undermining future food security. In: Fahad S, Hasanuzzaman M (Eds.), Environment, climate, plant and vegetation growth. Cham: Springer International Publishing, pp. 17-61.
- Ncama K, Aremu OA, Sithole NJ (2022) Plant Adaptation to environmental stress: drought, chilling, heat, and salinity. In: Galanakis CM (Ed.), Environment and Climate-smart Food Production. Cham: Springer International Publishing, pp. 151-179
- Valone TF (2021) Linear global temperature correlation to carbon dioxide level, sea level, and innovative solutions to a projected 6 C warming by 2100. J Geos Environ Prot 9(03): 84-135.
- Hussain HA, Men S, Hussain S, Chen Y, Ali S, et al. (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9(1): 3890-3901.
- He T, Li C (2020) Harness the power of genomic selection and the potential of germplasm in crop breeding for global food security in the era of rapid climate change. Crop J 8(5): 688-700.
- Atlin GN, Cairns JE, Das B (2017) Rapid breeding and varietal replacement are critical to the adaptation of cropping systems in the developing world to climate change. Glob Food Sec 12: 31-37.
- Revilla P, Anibas CM, Tracy WF (2021) Sweet corn research around the world 2015–2020. Agronomy 11(3): 534-583.
- FAO (2025) FAOSTAT statistical database. Food and Agriculture Organization of the United Nations.
- Safian N, Naderi MR, Torabi M, Soleymani A, Salemi HR (2022) Corn (Zea mays) and sorghum (Sorghum bicolor (L.) Moench) yield and nutritional quality are affected by drought stress. Biocatal Agric Biotechnol 45: 102486.
- Yadav S, Modi P, Dave A, Vijapura A, Patel D, et al. (2020) Effect of abiotic stress on crops. Sustain Crop Prod 3(17): 5-16.
- Bhattacharya A, Bhattacharya A (2021) Effect of soil water deficits on plant-water relationship: A review In: Bhattacharya A (Ed.), Soil Water Deficit and Physiological Issues in Plants, pp. 1-98.
- Garcia-Caparros P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, et al. (2021) Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev 87: 421-466.
- Marchiosi R, dos Santos WD, Constantin RP, de Lima RB, Soares AR, et al. (2020) Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem Rev 19: 865-906.
- Meulmeester FL, Luo J, Martens LG, Mills K, van Heemst D, et al. (2022) Antioxidant supplementation in oxidative stress-related diseases: What have we learned from studies on alpha-tocopherol?. Antioxidants 11(12): 2322.
- Bheemanahalli R, Ramamoorthy P, Poudel S, Samiappan S, Wijewardane N, et al. (2022) Effects of drought and heat stresses during reproductive stage on pollen germination, yield, and leaf reflectance properties in maize (Zea mays). Plant Direct 6(8): e434.
- Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, et al. (2017) Drought induced changes in growth, osmolyte accumulation, and antioxidant metabolism of three maize hybrids. Front Plant Sci 8: 69.
- Liu H, Able AJ, Able JA (2022) Priming crops for the future: rewiring stress memory. Trends Plant Sci 27(7): 699-716.
- Abhinandan K, Skori L, Stanic M, Hickerson NM, Jamshed M, et al. (2018) Abiotic stress signaling in wheat-an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front Plant Sci 9: 734.
- Liu H, Able AJ, Able JA (2016) SMARTER de-stressed cereal breeding. Trends Plant Sci 21(11): 909-925.
- Naikoo MI, Dar MI, Raghib F, Jaleel H, Ahmad B, et al. (2019) Role and regulation of plants' phenolics in abiotic stress tolerance: An overview. In: Khan MIR, Reddy PS, Ferrante A, Khan NA (Eds.), Plant signaling molecules, Woodhead Publishing, pp. 157-168.
- Tiwari YK, Yadav SK (2019) High temperature stress tolerance in maize (Zea mays): Physiological and molecular mechanisms. J Plant Biol 62: 93-102.
- Fan Y, Ma C, Huang Z, Abid M, Jiang S, et al. (2018) Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum). Front Plant Sci 9: 805.
- Wang X, Xin C, Cai J, Zhou Q, Dai T, et al. (2016) Heat priming induces trans-generational tolerance to high temperature stress in wheat. Front Plant Sci 7: 501.
- Llorens E, González-Hernández AI, Scalschi L, Fernández-Crespo E, Camañes G, et al. (2020). Priming mediated stress and cross-stress tolerance in plants: Concepts and opportunities. In: Hossain MA, Liu F, Burritt DJ, Fujita M, Huang B (Eds.), In Priming-mediated stress and cross-stress tolerance in crop plants. Academic Press, pp. 1-20.
- Pissolato MD, Martins TS, Fajardo YC, Souza GM, Machado EC, et al. (2024) Stress memory in crops: what we have learned so far. Theor Exp Plant Physiol 36: 535-565.
- Galviz Y, Souza GM, Lüttge U (2022) The biological concept of stress revisited: relations of stress and memory of plants as a matter of space-time. Theor Exp Plant Physiol 34(2): 239-264.
- Mauch-Mani B, Baccelli I, Luna E, Flors V (2017) Defense priming: an adaptive part of induced resistance. Annu Rev Plant Biol 68(1): 485-512.
- Gallusci P, Agius DR, Moschou PN, Dobránszki J, Kaiserli E, et al. (2023) Deep inside the epigenetic memories of stressed plants. Trends Plant Sci 28(2): 142-153.
- Liu J, He Z (2020) Small DNA methylation plays a significant role in plant responses to abiotic stress and memory. Front Plant Sci 11: 595603.
- Yakovlev IA, Fossdal CG (2017) In silico analysis of small RNAs suggest roles for novel and conserved miRNAs in the formation of epigenetic memory in somatic embryos of Norway spruce. Front Physiol 8: 674.
- Hossain MA, Li ZG, Hoque TS, Burritt DJ, Fujita M, et al. (2018) Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: key regulators and possible mechanisms. Protoplasma 255(1): 399-412.
- Sher A, Sarwar T, Nawa, A, Ijaz M, Sattar A, et al. (2019) Methods of seed priming, in: Hasanuzzaman, M., Fotopoulos, V. (Eds.), Priming and pretreatment of seeds and seedlings: implication in plant stress tolerance and enhancing productivity in crop plants. Springer, Singapore, pp. 1-10
- Guo X, Zhi W, Feng Y, Zho G, Zhu G (2022) Seed priming improved salt-stressed sorghum growth by enhancing antioxidative defense. Plos one 17(2): e0263036.
- Hardegree SP (1996) Optimization of seed priming treatments to increase low-temperature germination rate. J Range Manage 49(1): 87-92.
- Galicia-Juárez M, Zavala-García F, Sinagawa-García SR, Gutiérrez-Diez A, Williams-Alanís H, et al. (2021) Identification of Sorghum (Sorghum bicolor (L.) Moench) Genotypes with Potential for Hydric and Heat Stress Tolerance in Northeastern Mexico. Plants 10(11): 2265.
- Tas T (2022) Physiological and biochemical responses of hybrid maize (Zea mays) varieties grown under heat stress conditions. PeerJ 10: e14141.
- Rodríguez-Salinas PA, Zavala-García F, Urías-Orona V, Muy-Rangel D, Heredia JB, et al. (2020) Chromatic, nutritional and nutraceutical properties of pigmented native maize (Zea mays L.) genotypes from the northeast of Mexico. Arab J Sci Eng 45: 95-112.
- Nikolaeva MK, Maevskaya SN, Shugaev AG, Bukho NG (2010) Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ J Plant Physl 57: 87-95.
- Khan MN, Zhang J, Luo T, Liu J, Rizwan M, Fahad S, et al. (2019) Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits, and chloroplast ultrastructure perseveration. Ind Crop Prod 140: 111597.
- Rafiq M, Saqib M, Jawad H, Javed T, Hussain S, et al. (2023) Improving quantitative and qualitative characteristics of wheat (Triticum aestivum ) through nitrogen application under semiarid conditions. Phyton-Int J Exp Bot 92(4): 1001-1017.
- Zafar M, Ahmed S, Munir MK, Zafar N, Saqib M, et al. (2023) Application of Zinc, Iron and Boron enhances productivity and grain biofortification of Mungbean. Phyton-Int J Exp Bot 92(4): 983-999.
- Kosar F, Akram NA, Ashraf M (2015). Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum) seedlings under water stress. S Afr J Bot 96: 71-77.
- Saud S, Fahad S, Yajun C, Ihsan MZ, Hammad HM, et al. (2017) Effects of nitrogen supply on water stress and recovery mechanisms in Kentucky bluegrass plants. Front Plant Sci 8: 983.
- Liang B, Ma C, Zhang Z, Wei Z, Gao T, et al. (2018) Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ Exp Bot 155: 650-661.
- Bashir T, Naz S, Bano A (2020) Plant growth promoting rhizobacteria in combination with plant growth regulators attenuate the effect of drought stress. Pak J Bot 52(3): 783-792.
- Sharma A, Wang J, Xu D, Tao S, Chong S, et al. (2020) Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis Sci Total Environ 713: 136675.
- Rivas R, Falcão HM, Ribeiro RV, Machado EC, Pimentel C, et al. (2016) Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S Afr J Bot 103: 101-107.
- Luqman M, Shahbaz M, Waraich EA (2023) Effect of different concentrations of GR24 as seed priming treatment on physio-chemical and yield related attributes of maize (Zea Mays) hybrids under drought stress. Pak J of Bot 55(4): 1257-1266.
- Zhao Q, Ma Y, Huang X, Song L, Li N, et al. (2023) GABA application enhances drought stress tolerance in wheat seedlings (Triticum aestivum). Plants 12(13): 2495.
- Ru C, Hu X, Chen D, Wang W (2023) Droughts and thermopriming enhance acclimation to later drought and heat stress in maize seedlings by improving leaf physiological activity. Agronomy 13(4): 1124.
- Hussain M, Farooq M, Lee DJ (2017) Evaluating the role of seed priming in improving drought tolerance of pigmented and non‐pigmented rice. J Agron Crop Sci 203(4): 269-276.
- Sen A, Puthur JT (2020) Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa Physiol Mol Biol Plants 26(3): 551-565.
- Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90(5): 856-867.
- Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 10(2): 277.
- Syvertsen JP, Garcia-Sanchez F (2014) Multiple abiotic stresses occurring with salinity stress in citrus. Environ Exp Bot 103: 128-137.
- Nawaz M, Sun J, Shabbir S, Khattak WA, Ren G, et al. (2023) A review of plants strategies to resist biotic and abiotic environmental stressors. Sci Total Environ 900: 165832.
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5: 9-19.
- Scagel CF, Lee J, Mitchell JN (2019) Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind Crop Prod 127: 119-128.
- Gharibi S, Tabatabaei BES, Saeidi G, Talebi M, Matkowski A (2019) The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochemistry 162: 90-98.
- Rao MJ, Ding F, Wang N, Deng X, Xu Q (2018) Metabolic mechanisms of host species against citrus Huanglongbing (Greening Disease). Crit Rev Plant Sci 37(6): 496-511.
- Rao MJ, Xu Y, Huang Y, Tang X, Deng X, et al. (2019) Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol 19(1): 1-13.
- Rao MJ, Ahmed U, Ahmed MH, Duan M, Wang J, et al. (2021) Comparison and quantification of metabolites and their antioxidant activities in young and mature leaves of sugarcane. ACS Food Sci Technol 1(3): 362-373.
- Ahmed U, Rao MJ, Qi C, Xie Q, Noushahi HA, et al. (2021) Expression profiling of flavonoid biosynthesis genes and secondary metabolites accumulation in Populus under drought stress. Molecules 26(18): 5546.
- Park YJ, Kwon DY, Koo SY, Truong TQ, Hong SC, et al. (2023) Identification of drought-responsive phenolic compounds and their biosynthetic regulation under drought stress in Ligularia fischeri. Front Plant Sci 14: 1140509.
- Samota MK, Sasi M, Awana M, Yadav OP, Amitha Mithra SV, et al. (2017) Elicitor-induced biochemical and molecular manifestations to improve drought tolerance in rice (Oryza sativa) through seed-priming. Front Plant Sci 8: 934.






























