Review of Major Congenital Malformation in Cattle: Its Causes and Future Prospective to Reduce its Occurrence.
Nagaro Damana Begna1 and Abriham Kebede Deresa2*
1Wollega University, Horro guduru Dairy farm professional, Ethiopia
2Department of Veterinary Medicine, School of Veterinary Medicine, Wollega University, Oromia state, Ethiopia
Submission: August 09, 2023; Published: August 21, 2023
*Corresponding author: Abriham Kebede Deresa, Department of Veterinary Medicine, School of Veterinary Medicine, Wollega University, Oromia state, Ethiopia. E-mail: abrahamkebede2016@gmail.com
How to cite this article: Nagaro Damana Begna and Abriham Kebede Deresa*. Review of Major Congenital Malformation in Cattle: Its Causes and Future Prospective to Reduce its Occurrence.. Dairy and Vet Sci J. 2023; 15(5): 555925. DOI: 10.19080/JDVS.2023.15.555925
Summary
Malformations or congenital deformities are defined as abnormalities of structure present at birth. The cause of many congenital defects is unknown, but some are inherited. Also, another cause is list of recognized environmental causes which are maternal nutritional deficiencies, teratogenic drugs or chemical exposures, mechanical interference with the fetus, some viral infections, toxic plants, radiology, rectal palpation for gestation diagnosis and toxic effects of any kind that dam would be exposed to during the early stage of organogenesis. The major congenital malformations frequently occurred in calves are dwarfism, hydrocephalus, osteopetrosis, hypotrichosis, arthrogryposis, polydactyly, syndactyly, bulldog, photosensitivity, double muscling, parrot mouth, cryptorchidism, prolonged gestation and oculocutaneous hypopigmentation.
There are many undesirable traits that show up in cattle. These range from poor performance and structural unsoundness to semi-lethal and lethal diseases. Congenital defects are present in all breeds of cattle. In most herds, they are rather uncommon; however, occasionally the frequency within a herd will be high enough to be of considerable economic importance. Cytogenetic and molecular analysis can be used to identify the cause of birth defect. Good monitoring and control measures by seedstock operations will help control genetic defects in commercial cattle populations. Even with seed stock level management of genetic defects, commercial cattle producers may still need to cull carrier animals within their herds. Gap in research leads to a delay in reporting of the gene associated with the birth defect and limits our ability to establish mechanisms behind these diseases and advance scientific knowledge. Therefore, this paper outlines an approach to review major congenital malformations in cattle.
Keywords: Cattle; Causes; Congenital Malformations, Management.
Abbreviations: AI: Artificial insemination; CA: Congenital Anomalies; ASA: American Simmental Association; DM: Double Muscling; MFD: Mule Foot Disease; OS: Osteopetrosis; CSF: Cerebrospinal Fluid
Introduction
Congenital Malformations or congenital deformities are defined as abnormalities of structure and function present at birth. These range from poor performance and structural unsoundness to semi-lethal and lethal diseases. Such abnormalities can be caused by genetic (chromosome or gene mutation) or environmental (teratogenic chemical physical agents, infections, etc.) factors, as well as by their combinations [1]. They have been identified in several livestock species, including bovids [2]. Since Artificial insemination is (AI) is commonly used in cattle breeding, the identification of the gene mutations responsible for Congenital Malformation is a very important diagnostic issue which facilitates the eradication of AI bulls carrying a deleterious mutation. In this species, several causative chromosome mutations [3] as well as gene mutations causing skeletal malformations e.g., complex vertebral malformations or brachy spina were identified [4] (Figure1).
The genetic analysis of a single case of a malformed newborn usually starts with a cytogenetic | study focused on searching for chromosome abnormalities. It is known that an abnormal set of autosomal chromosomes (aneuploidies) are responsible for severe, usually lethal, congenital malformations, as was shown very recently in a stillborn calf with a trisomy of chromosome 29 [5]. Abnormal chromosome complement, manifested by the presence of a small supernumerary marker chromosome, can also be associated with congenital malformation, as it was widely documented in humans [6]. Cytogenetic analysis can also reveal chromosome instabilities which indicate a possible deleterious effect of teratogenic agents during intrauterine life. Such observations were reported in calves with polymedia or Amelia [7].
Strong inbreeding in the bovine population has increased the risk of the occurrence of genetic diseases. In fact, the wide use of only a few elite sires has enhanced the probability of the coupling of two mutated recessive genes in the genotype of animals [8]. Congenital abnormalities are an economic burden to the dairyman. Every abnormal calf is one less replacement for cows that leave the herd. In addition, the birth of an abnormal calf may be accompanied by severe dystocia [9]. Finally, good monitoring and control measures by seedstock operations will help control genetic defects in commercial cattle populations. Even with seed stock level management of genetic defects, commercial cattle producers may still need to cull carrier animals within their herds if the incidence rate of a genetic defect rises to a level where it becomes an economic problem. In most cases, careful sire and breed selection can be an effective approach for managing genetic defects in commercial herds without the need for extensive cow herd culling [10].
Despite the presence of congenital defects in all breeds of cattle and several genetic mutations, such as developmental duplication in cattle; there is scarcity of publication on it. This gap in research leads to a delay in reporting of the gene associated with the birth defect and limits our ability to establish mechanisms behind these diseases and advance scientific knowledge. Therefore, this paper outlines an approach to review major congenital malformations in cattle with the objectives of
Causes and types of congenital malformations in cattle.
Economic impact of congenital malformations in cattle.
How to manage congenital malformations in cattle.
Major Congenital Malformations in Cattle
Definition
Congenital anomalies (CA), or birth defects, are structural, behavioral, functional, and metabolic disorders that occur during intrauterine life and can be evident at birth or become manifest later in life [11].
Causes Of Congenital Malformation
The cause of many congenital defects is unknown, but some are inherited. Inherited disorders in cattle are mostly caused by autosomal recessively inherited genes. It is characteristic that the action of autosomal recessively genes only become expressed as a diseased phenotype if present in both loci. Therefore, autosomal recessively inherited disorders are of greater concern in cattle breeding than are disorders with dominant inheritance or recessive X-linked inheritance. As dominance or recessive X-linked genes are expressed in the phenotype of males, sires carrying such genes are mostly omitted from breeding. However, if the defective allele produces a desirable phenotype in heterozygous individuals, such animals may be used for breeding [12].
Congenital malformations are also caused by environmental problems. Causes included in the list of recognized environmental causes are maternal nutritional deficiencies, teratogenic drugs or chemical exposures, mechanical interference with the fetus, some viral infections, toxic plants, radiology, rectal palpation for gestation diagnosis and toxic effects of any kind that dam would be exposed to during the early stage of organogenesis [13].
Environmental Teratogens
Teratogens are factors identified as causing fetal anomalies. Teratogens usually do not affect the pregnant dam but damage the embryo/fetus primary during the first 40 days. Teratogens reported in cattle include toxic plants, virus, drugs, trace elements, and physical agents such as irradiation, hyperthermia, and undue pressure during rectal examination in early pregnancy. Teratogens follow seasonal patterns or known stressful conditions, may be linked to maternal disease, but do not follow a familial pattern [13].
Toxic plants
Ingestions of lupines (L. sericeous, L. caudates and L. nootkatones) between days 40-70 of gestation may cause joint contractures in calves associated with torticollis, scoliosis, and/ or kyphosis and various degree of cleft palate. Keelers and others at logan, Utah identified antipyrine as the teratogenic principle in lupines [14].
Viral infections
Prenatal viral infections may be teratogenic in cattle. Intrauterine Akabane virus infection caused abortion, premature birth, and congenital defects (arthrogryposis and hydranencephaly). Bovine viral diarrhea virus induced cerebellar dysplasia, ocular defects, brachygraphic, intrauterine growth retardation, and impaired immunologic competence [15]. Experimental transmission of bluetongue virus to pregnant heifers via insects resulted in abortion, stillbirths, and congenital defects such as arthrogryposis, campylognathia, and progathia with domed cranium [16].
Physical:
Early rectal palpation has an impact on the normal development process of the fetus. For example, rectal palpation between days 35 to 40 gestation has been reported to cause atresia coli [17].
Genetic factors
Genetic defects are pathophysiologic results of mutant genes or chromosomal aberrations occurring in any environment. Several congenital defects are inherited, mainly as simple autosomal recessives. At least 260 visible traits and defects of cattle have been reported to be controlled by major genes; most are pathologic, usually congenital, and are important to the cattle breeding industry. Diagnosis of defects due to genetic factors is necessary before effective control can be established. In Kansas for many years, a systematic program for gathering, recording, interpreting, and communicating information on congenital defects has been maintained [18]. As a rule, genetic defects determined by mutant gene(s) run in families and occur in typical intergenerational patterns and intergenerational frequencies requiring enumeration of normal and abnormal calves of a bull and identifying familial relationships. Breeding trails are necessary to confirm the genetic pattern of transmission of a defect, by conventional methods or by super ovulation of homozygous affected or heterozygous animals, embryo transplantation and pre terminal cesarean section where applicable [18).
Chromosome Defects
Although chromosomal aberrations are diagnostically important in man, their frequency and significance in cattle have yet to be ascertained. The most common chromosomal defects in cattle are 1/29 Robertsonian translocation and 14/20 translocation [19].
Major Types of Congenital Malformations
Though there are many congenital and inherited defects reported in cattle but some of the most occurred are discussed below [12].



Water Head (Internal Hydrocephalu)
Hydrocephalus is a multifactorial, congenital, or acquired disorder characterized by an abnormal accumulation of cerebrospinal fluid (CSF) within the cranial cavity. It is defined as internal when the CSF is accumulated in the ventricles and external when CSF is accumulated in the subarachnoid space [20]. The increase of CSF is, above all, related to its abnormal reabsorption or defective lymphatic drainage and rarely to its production, and it induces progressive enlargement of the head. Etiology includes genetic factors, developmental anomalies, intrauterine or prenatal infection, exposures to utero teratogens exposures, dietary deficiencies (vitamin A deficiency in the rabbit), or tumors or bleeding in the brain. The histopathological examination of this last case revealed the presence of cystic cavities in the thalamus and the substitution of nervous tissue with liquid; moreover, the cortex was thinner. [21] described a case of hydrocephalus in a male “Bull dog” calf. [22] described the case of dystocia due to hydrocephalic monster as a buffalo calf characterized by an extraordinarily large, football shaped head; on dissection, cranial bones were found markedly thin. Although genetic factors are the most frequent cause of this malformation, the authors have not fully evaluated intra-uterine infections and nutritional factors. Thus, the causes cannot be ruled out in this case.
Correale and Consalvo [23] have found three cases of hydrocephalus with typical alterations consisting of dilation of cerebral ventricles due to abnormal liquor accumulation and consequent increase of cranium volume. The nervous structures were reduced due to the pressure caused by the increased liquor volume; the calves were born dead or died within a few days after birth. No other malformations are described in those three cases and the possible action of infections and teratogen substances, such as drugs or toxins, is not supposed by the authors. This lets us assume that, in all the cases, a genetic cause is the most likely to cause.
Dwarfism
This is one of the most popular defects among beef cattle. There are a few types of dwarfism but all of them can be divided into two groups which are proportionate and disproportionate. The first one, also called pituitary or antibiotic dwarfism, is distinguished by small but proportionate stature. In most cases this kind of malformation is associated with hypothalamic growth hormone insulin-like growth factor axis. Formation of bone and soft tissue are reduced which results in infantile phenotype. Second one includes chondrodysplasia, chondrodystrophy and achondroplasia which may be manifested by disproportion in limbs changes in cranial development (flat and broad head), altered endochondral ossification, excessive amount of soft tissue due to its unaffected growing [24, 25] (Figure 2&3).
Different types of dwarfism are responsible for different genes and mutations. However, opinions are divided whether it is a disorder inherited in autosomal recessive manner or autosomal incomplete dominant manner. This is due to the phenomenon that heterozygous animals show symptoms of this defect. Besides carriers of this malformation, display features that are characteristic for some breeds and desirable for breeders (high meat yield). In most cases homozygous affected individuals are aborted or die right after birth, not infrequently because of decreased immunity [26].
Marble Bone (Osteopetrosis)
Osteopetrosis (OS) is a lethal autosomal recessive genetic defect previously identified in humans and a long list of animals. Cattle breeds known to be affected are Black and Red Angus, Hereford, Simmental, and Holstein. The defect was most recently reported in Red Angus cattle [27]. Calves affected by OS are born 10 to 30 days early. They usually have head abnormalities that consist of brachygnathia inferior, impacted molars, and a protruding tongue. The long bones are shorter than normal, the marrow cavities are filled with unabsorbed bone (primary spongiosa), but are very fragile, and can be easily broken [27] (Figure 4).

Samples from identified carriers were used to identify the mutation. The mutation is caused by a deletion of gene necessary for bone remodeling during development [28]. Genetic mutation that causes OS in Red Angus and Black Angus cattle are not the same, however, the mutation in Black Angus has not been identified. This could mean that the mutation in Black Angus changed or that they are 2 distinctly different mutations.
Rigid Joints (Arthrogryposis)
Arthrogryposis is a congenital disease characterized by nonprogressive joint contractures that can affect upper or lower limbs and/or the vertebral column, leading to various degrees of flexion or extension limitations, evident at birth. It is not a specific diagnosis, but rather a clinical finding of congenital contractures causing severe mobility difficulties in affected individuals. Arthrogryposis has been reported in livestock and pets and is often associated with muscle atrophy or other malformations. It has more than one etiological factor, including physical limitation of in utero movement causing fetal akinesia/hypokinesia syndrome, maternal illness, and intrauterine viral infection (Schmallenberg virus, Akabane virus, or Aino virus), toxin exposure, and genetic disorders affecting the fetus [29] (Figure 5).

Genomic deletion encompassing ISG15, HES4, and AGRN gene in the American Angus breed. [30] and in red dairy cattle it is associated with a deletion in the first axon of CHRNB1 [31] further more [32] in a piedmont calf affected by arthrogryposis, found the duplication of the SMN gene on BTA20q13.1 [32] In Southern [33] found a Murrah buffalo herd in which the disease was transmitted as an autosomal recessive disorder, moreover, in India [34] reported a case of a Murrah buffalo male fetus born dead with flexions and multiple articular rigidity of joints of all four limbs. Finally, Correale and Consalvo [23] observed a case of a calf born with an abnormal and permanent ankylosis of limb joints. In the cases described by Saini et al. [22] and Correale and Consalvo [23] the involvement of all four limbs let us assume gene abnormalities are responsible for the malformation.
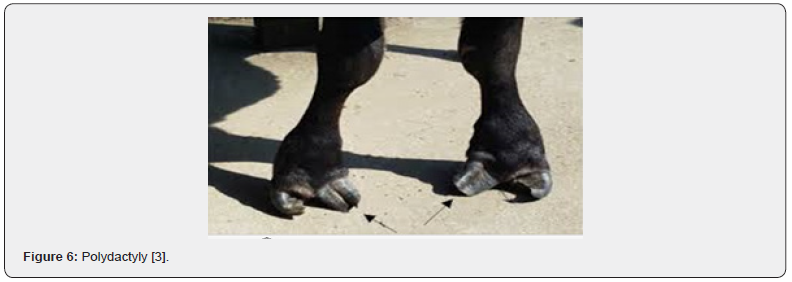
Extra Toes (Polydactyly)
Polydactyly is an abnormal condition in which cattle are born with one or more extra digits on one or more limbs. Polydactyly has been reported in several animal species. The condition occurs rarely in cattle but individual spontaneous cases (not produced by known polydactylous parent) have been reported sporadically for over a century. The condition has been observed in various cattle breeds. One or both front feet are usually affected, but all four may have the outer dewclaw develop into an extra toe. At least two sets of genes are involved in the inheritance of this trait [12] (Figure 6).
Mulefoot (Syndactyly)
Bone syndactylism is a heritable disorder also known as mule foot disease (MFD). This malformation has an autosomal recessive character, and it occurs differently in each case. This is due to incomplete penetrance and variable expression of this trait [35]. This genetic disorder is a non-division or fusion of digits, and it mostly appears as synostosis of phalanges. However, more proximal limbs might also be altered. Mulefoot disease might affect only one foot, as well as all 4 feet. Moreover, this defect is associated with hyperthermia resulting from increased environmental temperature [36]. Syndactylism can be present in both dairy and beef cattle. There have been noted cases of this defect in Holstein, brown Swiss, Simmental, Haryana, Angus, Hereford, Chanina, Japanese native, Swedish Red pied, Czech Black Pied, German Flockier, Danish cattle [37] (Figure 7).

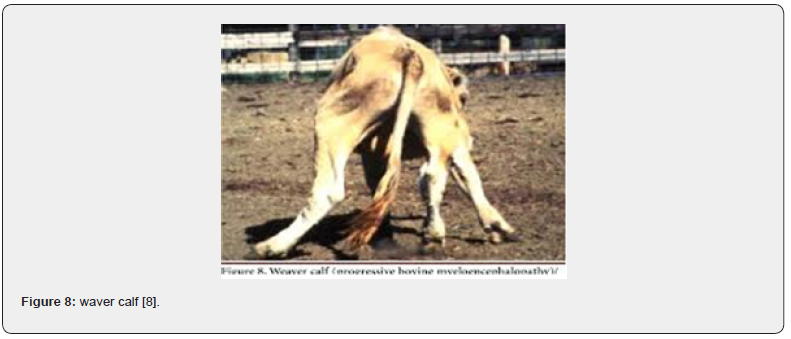
Weaver calf (progressive bovine Myel encephalopathy)
The disease occurs between six months and two years old and occasionally later. The main clinical signs are ataxia, progressive weakness of the pelvic limbs, difficulties to stand up, proprioceptive deficit and oscillatory hypermetric walking. The mental state is always alert and all the reflexes are always present and normal. This defect is inherited as a simple recessive trait [38] (Figure 8).
Photosensitivity (protoporphyria)
Animals are sensitive to sunlight and develop scabs and open sores when exposed to sunlight. The liver is also affected, and the animals may suffer from seizures. It is inherited as a simple recessive trait. Photosensitivity appears in individuals affected by this defect, which causes ulceration, alopecia, (mostly nostrils and earlobes lesion), and pain. Next to this symptom there can arise liver damage and reduction in productivity [39] (Figure 9).

Bulldog (chondrodysplasia)
This trait is inherited as an incomplete dominant. The homozygous may be aborted dead at 6-8 months gestation and has a compressed skull; nose divided by furrows and shortened upper jaw, giving the bulldog facial appearance. The heterozygous calf is small and heavy muscled. A calf affected with chondrodysplasia has short legs, large round head, cleft palate, internal hydrocephalus, and disturbed bone growth. Chondrodysplasia is a defect of interstitial growth of cartilage causing bone to be short and disorganized [40] (Figure 10).
Double muscling
It is the result of a defect in the myostatin gene, which is responsible for regulating the growth of muscle fibers during development. Without a functioning myostatin gene, muscle will develop hypertrophy (increase in muscle fiber size) and hyperplasia (increase in number of muscle fibers), resulting in the appearance of a” double muscled” animal. It is inherited as a simple recessive trait. Muscular hyperplasia, also known as double muscling (DM), is an inherited condition that results from an increase in several muscle fibers. This condition is also incorrectly called hypertrophy [41]. It was first reported by Culley in 1807 and then by Youth in 1834 [42] (Figure 11).
The occurrence of disease is observed in many beef cattle breeds including Belgian blue [43]. Animals with DM phenotype present many clinical syndromes. They are characterized by extreme high carcass yield, what relates to reduction in the size of most vital organs such as heart, lungs, kidneys, as well as high frequency of broken bones. Whereby, they are more susceptible to respiratory diseases, urolithiasis, alveolar hypoxia, hypoxemia, and dystocia in comparison to normal cattle. Moreover, endurance of double muscled cattle is less than in normal animals, leading to quick exhaustion after severe exercise, because it is less associated with reduction of mitochondrial gene expression [44].

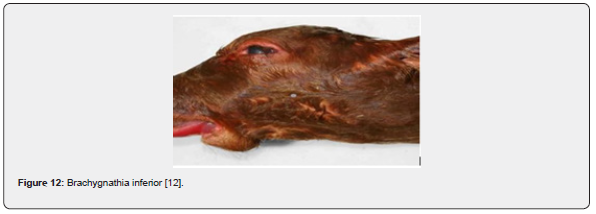
Parrot mouth (brachygnathia inferior)
It is abnormally short lower jaw and can be mandibular brachygnathia, which is also known as brachygnathia inferior and commonly called parrot mouth or overbite, is characterized by failure of the anterior of the lower jaw forward of the premolars to grow to normal length. Brachygnathia superior is characterized by failure of the premaxillary bone to grow to normal length and width. Aside from studies concerning the disruption of thyroid hormone function during development [45], brachgnathia superior and other developmental malformations commonly associated with fetal thyroid dysfunction are increasing in calves [46] (Figure 12).

Because facial malformations also were observed at increasing frequency, malformations of the upper and lower mandibles were quantified. Neither brachygnathia superior nor maxillary brachygnathia commonly cause mortality postnatally, so they can be observed on individual of both sexes and all age groups. However, facial malformations likely compromise feeding efficiency and therefore increase probability for starvation during winter and may reduce growth rate in calves. Moreover, experimental research has revealed that malnourished domestic cow produce offspring with compromised thyroid function [47]
Cryptorchidism

A condition in which one or more testicles fail to descend into the scrotum and it is an abnormality in the development of phenotypic sex without sexual ambiguity. In addition to environmental factors, like endocrine disruptors, cryptorchidism is at least in part determined by genetic causes and, until now, many candidate genes have been identified [48]. It is very common in livestock and pets (Figure 13).
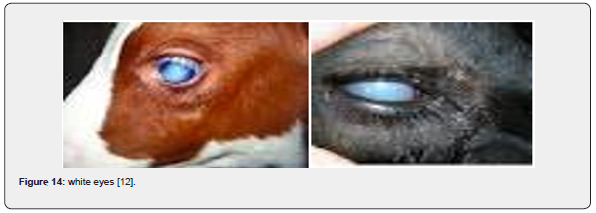
White eyes (oculocutaneous hypopigmentation)
Oculocutaneous hypopigmentation was originally reported to the American Simmental Association (ASA) in 2012, where a calf was reported with “white colored eyes and a diluted hair coat”. Over the next two years, three additional calves were reported to the ASA with similar characteristics. As all the appropriate DNA samples had been collected and stored, an investigation was conducted and identified the abnormalities were a result of a genetic mutation. Subsequent DNA testing identified that the mutation was most likely introduced into the Black Simmental breed from Angus cattle during the development of black purebreds. The hair coat is a bleached color, and the iris is pale blue around the pupil with tan periphery (Figure 14).
Economic Importance of Abnormal Development
The obvious impact of abnormal development is loss of the nonviable fetus. This presents two possible problems for the producer: first, financial loss, through loss of saleable animals and the cost of retaining the dam for another year in the case of cattle; second, this abnormal fetus may herald an outbreak of similarly affected fetuses if the whole herd has been affected by an environmental teratogen or if an abnormal gene has entered the gene pool of the herd [12].
Methods of identifying causes of defects
When an abnormal calf is born, first you should try to determine if the defect is hereditary. Congenital defects that are environmentally caused rather than inherited often occur during a short period in a group of cattle that are managed the same way. Malnutrition, toxic factors, infectious disease, or extreme weather may be to blame for these congenital defects. When a pregnant cow consumes the lupine plant between days 40 to 60 of gestation, her calf may be born with crippled-calf (crooked-calf) disease, a crooked-leg condition. Calves with extra legs may be the result of fetal development mistakes. Bovine Virus Diarrhea (BVD) infection during pregnancy can lead to some hydrocephalus. Flexed pastern can be caused by a large fetus developing in a small uterus. Each of these conditions may at first appear to be genetic defects but are caused by environmental conditions. In the case of flexed pasterns, the causes could be either environmental or genetic. Once it is determined that a specific environmental cause is responsible for a defect, management changes can be put in place to address these problems and reduce the risk of future incidence [49].
Genetic tests for DNA markers can usually help identify inherited defects. Genetic tests for simple traits that are controlled by one gene can accurately assess whether an animal is a carrier (heterozygous, two different alleles) or homozygous (two same alleles) for marker of a certain phenotype (physical manifestation of a trait). Breed associations and genetic testing companies can provide testing protocols for genetic defects associated with a certain breed. If a defect is a dominant, no test is needed because the animal would display the defect even if only one dominant allele was present. A small number of congenital defects are caused by genes with incomplete dominance, but some are caused by two or more sets of genes. If the defect is inherited as incomplete dominance, an animal that has only one undesirable allele can usually be identified, and testing is not needed. Genetically caused defects tend to run in families. The sire and dam of a calf will likely have at least one common ancestor. The occurrence of multiple affected calves in a herd often results from the same sire and closely related dams [7].
Breeders must have good records to determine the cause of defects. These records must include calf percentage, description of abnormalities, photos, or videos of the abnormalities when possible, and causes of death. If percentage is not known, from records, it can be determined with DNA testing. Necropsies are valuable for investigating possible causes of death and ties to congenital defects. Management groups and movement among paddocks or pastures should be recorded as well. Feed and forage analysis reports, notes of toxic plant presence, and herd health records help in determining the cause of any congenital abnormalities [49].
Managing Congenital Defects
Once carrier and affected animals are identified, producers can make selective breeding and culling decisions to manage a genetic defect within a herd. When a carrier animal is extensively used for breeding purposes, as in the case of sires for artificial insemination purposes, several thousand calves may be sired by the carrier bull before the abnormality expresses itself. Even more mating using the carrier sire may take place before affected calves are associated with the sire and genetic testing confirms the sire as a carrier. In some cases, a genetic test may not be available during the initial occurrence of the condition. As a result, a diagnostic test may need to be developed based on data submitted from producers who observe the defect in their herds. Therefore, it is important to test sires whose semen will be marketed. It is also important to test donor dams in embryo transfer programs. Furthermore, producers must remain vigilant about observing calf crops for congenital defects by collecting appropriate records and animal samples for diagnostics. They should report problems with occurrence of defects to breeding animal suppliers and breed associations. Consider the value of a carrier’s genetic worth to the breeding program [10].
An animal with one undesirable recessive gene may also have thousands of very desirable genes. Carriers that have superior genetics should be strategically paired with non-carriers in a terminal crossbreeding program where all calves are marketed for beef production and not for breeding purposes. Alternatively, a superior son could be used as a herd sire that does not carry the defect. In most cases, defective carriers should not be used to produce breeding animals; therefore, do not keep replacement heifers that are defective carriers. When you can utilize other cattle with similar or superior genetic merit that do not carry the defect, you should systematically work carrier females out of the herd and replace them with cattle that do not carry the defect. Be sure to send carriers directly to harvest to avoid transferring the defect to another breeding herd [50].
Purebred breeders and breed associations share responsibility for controlling genetic defects in seed stock populations. In seed stock herds, use available DNA diagnostic tools to test suspect animals or those known to have ancestors that are carriers. When carriers are retained in the breeding herd, test all progeny to determine carrier status before marketing them as breeding animals. Seed stock producers have an obligation to be honest and notify the customers they supply with breeding animals as well as their respective breed associations when carriers of genetic defects become known. Many breeds associations mandate genetic defect reporting among their members. Breeders should photograph any calf born with a suspected defect and then contact the breed association to arrange for tissue collection and reporting. Breed associations may also have rules regarding registration eligibility or required denotations on registration papers for animal carrying or affected with genetic defects. Make sure buyers understand the consequences of using offspring from known carriers. Serious ethical and legal problems can be involved in marketing known carrier cattle or progeny of known carriers. Marketing carriers without informing the buyer can not only harm breeder reputation but may also reflect negatively on the entire breed [49].
Current Status and Future Perspective of Congenital Defects in Cattle
Congenial defects are highly increasing in cattle due to different factors like extensive breeding activities with poor seedstock selection, increased application of reproductive biotechnology with poor breeding policy, etc. molecular genetics in livestock has been subject to extensive study during the last two decades to minimize the congenital malformation. The international community has made stride both in advocacy and in implementation of specific actions aimed at reducing the impact of birth defects using different approaches. Now researchers are investigating different methods to reduce their further occurrence in the herd by genetic improvement program. These studies are related to gene-based selection of mendelian traits (mainly diseases and genetic defects), marker assisted selection and introgression. Furthermore, molecular information is increasingly used to assist breed conservation Programs and to improve understanding of the origin and domestication of livestock [51]. In the current time, different practices are being used to minimize congenital malformation in cattle.
Gene-based selection
Increasing knowledge of the animals’ genome increases the prospects for applying this technology and provides new tools with which to select for healthy animals. Initial applications are related to mendelian traits. In cattle for example, DNA diagnosis is routinely utilized to eliminate genetic disorders such as bovine leukocytes adhesion deficiency (BLAD) deficiency of uridine monophosphate synthase (DUMPS) and complex vertebral malformation (CVM), as well as in selection for traits such as milk kappa-cesein and double muscling [52].
Marker assisted selection.
Most economically important traits in animal production are of a quantitative nature and are affected by many genes (loci), a few of which have major effects, while the majority have small effects [53,54]. If a gene (locus) with a major effect can be identified, and if a molecular test can be designed, animals’ genotypes at the locus can be used for selection. In other cases, a chromosomal region close to the gene of interest may be identified and used as a marker. Mixed models of inheritance, which assume one or several identified segregating loci, and an additional polygenic component, have been developed. When genotypes at each identified locus are known, they can be treated as fixed effects in standard mixedmodel techniques [55]. When only genotypes at linked markers are known, the uncertainty resulting from unknown haplotypes and recombination events must be considered [56].
Extra genetic gain is usually to be expected if information on genes with medium to large effects is included in the genetic evaluation process. Numerous studies have investigated this problem in recent years. Results are not always comparable, because selection criteria differed between studies (i.e., from an index based on individual information to animal models), but they all indicate that knowledge of genotype at quantitative trait loci generally improves short-term response to selection [57]. In less favorable situations where only genotypes at linked markers are known, results largely depend on the circumstances. Large gains can be expected when linkage disequilibrium exists at the population level [58], and when traits are difficult to measure (e.g., disease resistance), sex limited (e.g., traits related to egg or milk production), expressed late in the lifespan of the animals (longevity and persistency in litter size), or measure after slaughtering (e.g., meat quality traits).
In other cases, the advantage of marker assisted selection may be questionable. Genes at the same or at different loci interact with each other in producing a phenotypic effect. It is seldom known how this occurs. When, by using statistical models, an apparent effect is assigned to a particular gene, such interaction is not considered. This explains, at least partly, why even when genes with major effects are identified, incorporating them (or their markers) into a selection program may not achieve the desired results. Because of such interactions, there is often an apparent lack of consistency between different studies related to the use of genetic markers [59]. To correctly assess the effect of a gene, the average effect over the possible genotypes in the production where the information is to be applied weighted according to their frequencies) must be considered.
Introgression
Introgression is advocated mainly to improve disease resistance in each population. If markers for the resistance gene(s) (or probe for the gene) are available, marker assisted selection may be used to simplify the process of introgression. [60] discuss the use of repeated backcrosses to InProgress a gene into a population. If the non-resistant breed is considered the recipient breed, and the breed that carries the resistance gene is considered the donor breed, introgression of the desirable gene from the donor breed to the recipient breed is accomplished by the multiple backcrosses to the recipient breed, followed by one or more generations of intercrossing. The aim of the backcross generations is to generate individuals that carry one copy of the donor gene, but that are like the recipient breed for the rest of the genome. The aim of the intercrossing phase is to fix the donor gene. Marker information can enhance the effectiveness of the backcrossing phase of gene introgression strategies by identifying carriers of the target gene (foreground selection), and by enhancing recovery of the recipient genetic background (background selection).
Generally, it is more feasible and economically sound to mate, in successive generations, pure-bred females of the recipient breed to cross-bred males that carry the desired gene, than to carry out the reverse process. If the gene for resistance is dominant, its introgression into a population may be effective even without a molecular marker for the gene. If the gene for resistance is recessive (or co-dominant), markers are necessary. In case where resistance is polygenic, introgression without genetic markers is not likely to be effective; by the time the genetic influence of the donor breed is high enough to give high levels of resistance, the desired characteristics of the recipient breed will probably have been lost [61].
Conclusion and Recommendations
Congenital defects which can be caused by genetic (chromosome or gene mutations, which may be due to strong inbreeding) or environmental (teratogenic chemical or physical agents, infections, etc.), have financial loss, through loss of saleable animals and the cost of retaining the dam for another year in the case of cattle; this abnormal fetus may herald an outbreak of similarly affected fetuses if the whole herd has been affected by an environmental teratogen or if an abnormal gene has entered the gene pool of the herd. [62] Identification of the etiology of developmental anomalies is extremely difficult for many reasons. Based on the above conclusions, the following recommendations are forwarded: -
In livestock farming system good monitoring and control measures by seedstock operations to control genetic defects should be applied.
Breeders must have good records to determine the cause of defects.
Once carriers and affected animals are identified, procedures should make selective breeding and culling decision to manage a genetic defect within a herd.
Avoid pregnant cattle accessing teratogens that cause congenital malformations.
Avoid inbreeding.
References
- Corsello G, Giuffrè M (2012) Congenital malformations. J Matern Fetal Neonatal Med 1: 25-29.
- Albarella S, Ciotol F, D’Anza E, Coletta A, Zicarelli L, et al. (2017) Congenital Malformations in River Buffalo (Bubalus bubalis). Animals(Basel) 7(2): 1-9.
- Dittmer KE, Thompson KG (2015) Approach to Investigating Congenital Skeletal Abnormalities in Livestock. Vet Pathol 52(5): 851-861.
- Charlier C, Agerholm JS, Coppieters W, Karlskov Mortensen P, Wanbo Li, et al. (2012) A Deletion in the bovine FANCI gene compromises fertility by causing fetal death and brachyspina. PLoS One 7(8): e43085.
- Häfliger IM, Seefried F, Drögemüller C (2020) Trisomy 29 in a still born Swiss Original Braunvieh calf. Anim Genet 51(3): 483-484.
- Liehr T, Mrasek K, Weise A (2006) Small supernumerary marker chromosomes-progress towards a genotype-phenotype correlation. Cytogenet Genome Res 112(1-2): 23-34.
- Szczerbal I, Kociucka B, Nowacka-Woszuk J, Lach Z, Jaskowski JM, et al. (2014) A high incidence of leukocyte chimerism (60, XX/60, XY) in single born heifers culled due to underdevelopment of internal reproductive tracts. Czech J Anim Sci 59(10): 445-449.
- Arcanglo G, Stefania T (2006) Inherited disorder of cattle: A Selected Review. Slov Vet Res 43(1): 17-29.
- Gilmore LO, Fecieimer NS, Herschler MS (1962) Congenital abnormalities in cattle and their association with hereditary and environmental factors. Journal of Dairy Science 45(12): 1493-1499.
- Field TG, Robert WT (2007) Beef Production and Management Decisions. 5th ed. Pearson Education, Inc. Upper Saddle River, NJ.
- Sadler TW (2015) Birth Defects and Prenatal Diagnosis. In: Langman’s medical embryology. 13th (ed). Philadelphia: Wolters Kluwer Pp: 126-140.
- Mamta J, Pandya GM, Dangar NS, Kharadi VB (2014) Congenital defects in cattle. Indian Farmer 1(1): 15-18
- Rafid M, Naeem H (2010) Congenital anomalies in cattle and buffalo within Mudaina city in Basrah province between period 2007- 2009. Kufa Journal for Veterinary Medical Sciences 1(1): 1-12.
- Keller RF (1978) Alkaloid teratogens from Lupinus, Conium Veratrum and related general. In: Effects of poisonous plants on livestock. RF Keeler. KR VanKampen, LF James, Eds. pp: 397, New York: Academic Press, USA.
- Hillman R (1990) Persistent infection with BVD virus. Proc. 13th NAAB Technical Conference Pp: 115-119.
- Erasmus BJ (1990) Blue Tongue Virus. In: Virus Infections of Ruminants. Z. Dint- er and B. Morein, Eds., 227- 232. Elsevier Science Publishers B.V. New York, USA.
- Schlegel F, Muller W, Wille S, Buscch W (1986) Die rektale Friihtrachtigkeitsuntersu- chung als auslosender Faktor der partiellen Kolonaplasie beim Rind. Mh Vet Med 41: 377-382.
- Leipold HW, Huston K, Dennis SM (1983) Bovine congenital defects. Adv Vet Sci Comp Med 27: 197-271.
- Mcfeeley RA (1991) The 14/20 translocation in Simmental cattle. Personal communication.
- Thomas WB (2010) Hydrocephalus in dogs and cats. Vet Clin N Am Small Anim Pract 40(1): 143-159.
- Saini GS, Pandey AK, Chaudhary RN, Kumar A, Sharma S (2010) Arthrogryposis in a Murrah buffalo calf: A case report. Buffalo Bull 29: 318-320.
- Sharma K, Joshi M, Khosa JS, Singh D (2015) An unusual case of dystocia due to hydrocephalic monster in a buffalo. Int J Sci Environ Technol 4(2): 300-304.
- Correale E, Consalvi F (2003) About some congenital malformation in buffaloes bred in the Salerno province (Italy). Bubalus Bubalis 9: 22-29.
- Latter MR, Latter BDH, Wilkins JF, Windsor PA (2006) Inheritance of proportionate dwarfism in Angus cattle. Aust Vet J 84(4): 122-128.
- Murgiano L, Jagannathan V, Benazzi C, Bolcato M, Brunetti B, et al. (2014) Deletion in the EVC2 gene causes chondrodysplastic dwarfism in Tyrolean Grey cattle. PloS One 9(4): e94861.
- Whitlock BK, Kaiser L, Maxwell HS (2008) Heritable bovine fetal abnormalities. Theriogenology 70(3): 535-549.
- Nietfeld J (2007) Osteopetrosis in calves. Periodical.
- Kaiser L (2009) The gene gurus have been busy this decade: Recessive defect, mutations, and DNA-based tests. Periodical.
- Agerholm JS, Hewicker-Trautwein M, Peperkamp K, Windsor PA (2015) Virus-induced congenital malformations in cattle. Acta Vet Scand 57(1): 1-54.
- Beever JE, Marron BM (2016) Screening for Arthrogryposis Multiplex in Bovines. Kansas Stockman. Kansas Livestock Association. Topeka, KS.
- Agerholm JS, McEvoy FJ, Menzi F, Jagannathan V, Drögemüller C (2016) A CHRNB1 frameshift mutation is associated with familial arthrogryposis multiplex congenital in red dairy cattle. BMC Genomics 17: 479.
- Iannuzzi L, Di Meo GP, Perucatti A, Rullo R, Incarnato D, et al. (2003) Comparative FISH-mapping of the survival of motor neuron gene (SMN) in domestic bovids. Cytogenet. Genome Res 102(1-4): 39-41.
- Schild AL, Soares MP, Damé MC, Portianski EL, Riet Correa F (2003) Arthrogryposis in Murrah buffaloes in southern Brazil. Pesq Vet Bras 23(1): 13-16.
- Pandey AK, Saini GS, Chander S, Chaudhary RN, Jakhar P, et al. (2010) Dystocia due to abnormal calf in a buffalo: A case report. Buffalo Bull 29: 315-317.
- Johnson EB, Steffen DJ, Lynch KW, Herz J (2006) Defective splicing of Megf7/Lrp4, a regulator of distal limb development, in autosomal recessive mulefoot disease. Genomics 88(5): 600-609.
- Yadegari M, Vahed E, Ashtari MS, Tavakol S, Khamesipour F (2013) Report of congenital syndactyly (mule foot) in cattle. Global Veterinaria 10(4): 464-466.
- Leipold HW, Schmidt GL, Steffen DJ, Vestweber JG, Huston K (1998) Hereditary syndactyly in Angus cattle. J Vet Diagn Invest 10(3): 247-254.
- Radostits OM (2002) b:Doenças causadas pela herança de caracteres indesejá In: Radostits, O.M. Clínica veterinária – Um tratado de de doenças de bovinos, ovinos, suínos, caprinos e eqüinos. Rio de Janeiro: Roca Cap 30 Pp: 1561-1593.
- Armstrong SC, Jonsson NN, Barrett DC (2002) Bovine congenital erythro- cytic protoporphyria in a Limousin calf bred in the UK. Vet Rec 150(19): 608-610.
- Jayo MJ, Horton WA, Leipold HW, Den- nis SM (1987) Bovine Dwarfism: Clinical, biomedical, radiological, and pathological aspects. J Vet Med (A), 34: 161-171.
- Bouyer C, Forestier L, Renand G, Oulmouden A (2014) Deep intronic mutation and pseudo exon activation as a novel muscular hypertrophy modifier in cattle. PLoS One 9(5): e97399.
- Short RE, MacNeil MD, Grosz MD, Gerrard DE, Grings EE (2002) Pleiotropic effects in Hereford, Limousin, and Piedmontese F2 crossbred calves of genes controlling muscularity including the Piedmontese myostatin allele. J Anim Sci. 80(1): 1-11.
- Grisolia AB, D’Angelo GT, Porto Neto LR, Siqueira F, Garcia JF (2009) Myostatin (GDF8) single nucleotide polymorphisms in Nellore cattle. Genet Mol Res 8(3): 822-830.
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, et al. (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle- specific knock-out animals. J Biol Chem 282(41): 30014-30021.
- Soto-Blanco B, Gornia SL (2004) Prenatal toxicity of cyanide in goats-a model for teratological studies in ruminants. Theriogenology 62(6): 1012-1026.
- Kacar C, Oezcan K, Takci I, Guerbulak K, Oezen H, et al. (2008) Diprosopus, craniorachischisis, arthrogryposis, and other associated anomalies in a stillborn lamb. J Vet Sci 9(4): 429-431.
- Rae MT, Rhind SM, Kyle CE, Miller DW, Brooks AN (2002) Maternal under nutrition alters triiodothryonine concentrations and pituitary response to GnRH in fetal calves. J Endocrinol 173(3): 449-455.
- Urh K, Kunej T (2016) Molecular mechanisms of cryptorchidism development: Update of the database, disease comorbidity, and initiative for standardization of reporting in scientific literature. Andrology 4(5): 894-902.
- Brandi KJ, Parish JA, Trent S (2019) Managing Genetic Defects in Beef Cattle herds. Mississippi State University, Publication 662: 325-5839.
- Good C (2010) Testing Helps Avoid Genetic Defects P: 28-29.
- Maci LM, Alison LV (2022) Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agriculture and Bioscience 3: 1-13.
- Smits MA, Barillet F, Harders F, Boscher MY, Vellema P, et al. (2000) Genetics of scrapie susceptibility and selection for resistance. In Proceedings of the 51st Meeting of the European Association for Animal Production (EAAP) pp: 21-24.
- Le Roy P, Naveau J, Elsen JM, Sellier P (1990) Evidence for a new major gene influencing meat quality in pigs. Genetical Research 55(1): 33-40.
- Andersson L, Haley CS, Ellegren H, Knott SA, Johansson M, et al. (1994) Genetic mapping of quantitative trait loci for growth and fatness in pigs. Science 263(5154): 1771-1774.
- Kennedy BW, Quinton M, van Arendonk JA (1992) Estimation of effects of single genes on quantitative traits. Journal of Animal Science 70(7): 2000-2012.
- Fernando RL, Grossman M (1989) Marker-assisted selection using best linear unbiased prediction. Genetics Selection and Evolution 21(4): 467-477.
- Larzul C, Manfkedi E, Elsen JM (1997) Potential gain from including major gene information in breeding value estimation. Genetics Selection Evolution 29(2): 161-184.
- Lande R, Thompson R (1990) Efficiency of marker- assisted selection in the improvement of quantitative traits. Genetics 124(3): 743-756.
- Rocha JL, Sanders JO, Cherbonnier DM, Lawlor TJ, Taylor JF (1998) Blood groups and milk and type traits in dairy cattle: After forty years of research. Journal of Dairy Science 81(6): 1663-1680.
- Dekkers JCM, Hospital F (2002) The use of molecular genetics in the improvement of agricultural populations. Nature 3: 22-32.
- Konno S, Nakagawa M, Moriwaki M (1982) Akabane disease in cattle: congenital abnormalities caused by viral infection. Spontaneous disease. Vet Patholm 19(3): 246-266.
- Marcolongo Pereira C, Schild AL, Pereira Soares M, Vargas SF, Riet Correa F (2010) Congenital defects in ruminants in southern Brazil. Pesq Vet Bras 30: 816-826.






























