Mitigating Aflatoxin Contamination in Grains: The Importance of Postharvest Management Practices
Tarek A El-Desouky
Department of Food Toxicology and Contaminant, National Research Centre, Dokki, Giza, Egypt
Submission:November 28, 2024; Published: February 11, 2025
*Corresponding author:Tarek A El-Desouky, Department of Food Toxicology and Contaminant, National Research Centre, Dokki, Giza, Egypt. Email: eldesoukyt@yahoo.com
How to cite this article:Tarek A El-D. Mitigating Aflatoxin Contamination in Grains: The Importance of Postharvest Management Practices. Adv Biotech & Micro. 2025; 18(4): 555995.DOI:10.19080/AIBM.2025.18.555995
Abstract
Grains are the most important food staples for humans and livestock animals. Grains are susceptible to fungal attacks while in the field and/or storage. The toxigenic fungi not only cause quality deterioration and grain loss but also produce toxic secondary metabolites. Known as mycotoxins, the most important ones regarding their occurrence and toxicity are aflatoxins (AFs), which can cause many bad effects on humans and animals ingested. AFs may cause acute or chronic diseases such as carcinogenic, mutagenic, teratogenic, and hepatotoxic. Numerous studies around the world indicate that the contamination of most grains with one or more types of AFs leads to large economic losses, such as food commodity rejection by many countries (EU authorities, for example). To protect human beings and animals from these health risks, many countries have established regulations to limit exposure to AFs, as well as several strategies and measures to mitigate both the presence and concentration of AFs. For that, the purpose of this state of art is to present the status of aflatoxin contamination at the local and global levels in the main grains (corn, wheat, and rice), along with the most important methods and strategies proposed to mitigate AFs, which can be divided into physical, chemical, and biological methods during post-harvest.
Keywords: Aflatoxins, Postharvest, Grains, Management, Mitigation, Mycotoxins
Abbreviations: AFs: Aflatoxins; TLC: Thin Layer Chromatography; JECFA: Joint Expert Committee on Food Additives and Contaminants; EU: European Union; RASFF: Rapid Alert System for Food and Feed; IGC: International Grains Council; UV: Ultraviolet; LAB: Lactic Acid Bacteria; AI: Artificial Intelligence
Introduction
Aflatoxins (AFs) are one of the most important mycotoxins produced by fungi, fundamentally by Aspergillus species, under specific conditions such as temperature, relative humidity during preharvest, post-harvest, transportation, and storage [1-3]. Four main types of AFs (B1, B, G1, and G2) have been identified. AFB1 has been reported to be carcinogenic, teratogenic, and mutagenic to a wide range of organisms and is known to cause hepatic carcinoma in humans [4,5]. The (IARC) has categorized AFB1 as the most dangerous human carcinogens known, placing them in Group 1 [5]. AFs incidence has been documented in many global regions. However, a higher occurrence of AFs is associated with tropical and subtropical areas and some temperate regions, which are now more susceptible to the presence of AFs due to climate change and poor practices during preharvest, harvest, and postharvest activities [6]. Methods for controlling AFs are largely preventive and include good agronomic practices such as using sound, fungus-free seeds for planting, controlling insects and plant diseases, and proper irrigation practices. These methods are essential to avoiding contamination of raw materials and processed products; therefore, they are an option to guarantee product safety for consumers [7]. Once the contamination by aflatoxins has occurred, other strategies post-harvest have been proposed to reduce the risk of exposure to AFs, which include physical, chemical, and biological removal or combining more than one method to achieve the target. In this state of the art, I will present the global status of the occurrence of aflatoxins in the three main grains (corn, wheat, and rice) for the biggest producers. Also, discuss the different strategies and how they work to reduce or mitigate AFs post-harvest.
Definition and Properties of Aflatoxins
Aflatoxins (AFs) are a group of secondary metabolites produced by many Aspergillus species, such as Aspergillus flavus, A. parasiticus, A. nomius, A. bombycis, A. pseudotamarii, and A. aflatoxiformans [2,8] Aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2) are thse four main AFs that are produced naturally. B and G refer to the blue and green, fluorescent colors produced under UV light on Thin Layer Chromatography (TLC) plates (Figure 1). The AFs molecule contains a coumarin nucleus linked to a difuran and either a pentanone, as in AFB1 and the dihydro derivative AFB2, or a sixmember lactone, as in AFG1 and its corresponding derivative AFG2. AFs are easily soluble in moderately polar organic solvents like methanol and chloroform, but only very weakly soluble in water and insoluble in non-polar solvents [9,10].

Conditions of fungal growth and AFs production on grains during post-harvest
Fungal growth and the production of AFs in the stored-grain ecosystem are influenced by several factors, such as temperature, water activity, relative humidity, substrate, and fungal strain. In addition, there are conditions related to storage that encourage the formation of AFs, such as gas concentration, time, and interaction with insects, as well as grain defects like physical and chemical damage that affect the production of AFs [11,12].
Impact of temperature, water activity (aw), and relative humidity
Temperature and water activity play a key role in the association of fungal growth with grains. Aflatoxigenic fungi can grow in a wide range of temperatures (19–35°C), with 28°C optimum for growth and 28–30°C for AFs production (Lahourar et al., 2016; [13,14]. AFs produced with optimal relative humidity (85%), while 95% relative humidity increases AFs production to a considerable level [15,16]. The moisture content and aw of the grain increase during storage if the relative humidity of the surrounding air is higher than the grain’s equilibrium relative humidity. A higher level of aw during storage makes grains more susceptible to fungus invasion, germination, growth, and AFs production [17].
Effect of fungal strains and pH on the production of AFs
Members of Aspergillus Section Flavi, which comprises 33 species, the majority of which are toxigenic (produce aflatoxin), are responsible for the production of AFs. Prominent toxigenic and economically important members of the section are A. flavus and A. parasiticus. According to [9], 18 of the 33 species in Flavi produce aflatoxins. In addition, 16 species could produce the four types (AFB1, AFB2, AFG1, and AFG2). The other two species, A. togoensis and A. pseudotamarii, produce either AFB1 alone or AFB1 and AFB2, respectively. Toxigenic fungi can grow in a wide range of pH (1.7-9.3), but the ideal range is (3-7). According to [18] the lower pH (3 > pH > 1) inhibition the growth and produce of AFs in contrast higher pH (6 > pH > 3) encourages to produce of AFs. In addition, light has an impact on the growth of fungi and formation of AFs. Whereas, darkness increases AFs production while sunlight inhibits it [19]. Short wave light and decreased water activity in the substrate work together to efficiently and persistently inhibit the growth of aspergilli that produce mycotoxin [20].
Substrate and their effect on AFs production
The substrate and other nutritional components like carbon, nitrogen, lipids, amino acids, and a few trace elements also have a significant impact on aflatoxin formation. Substrate rich in carbohydrates supports more production of AFs, as carbohydrate easily provides carbon, which is needed for good fungal growth [21]. Among carbohydrates, glucose, ribose, sucrose, xylose, and glycerol act as excellent substrates, while peptone, lactose, and sorbose were unable to promote AFs production [22]. Lipids are also necessary for the biosynthesis of AFs, such as lipophilic epoxy fatty acids, which cause ergosterol oxidation-induced AFs generation and fungal proliferation. Additionally serve as a substrate for acyl-CoA starting synthesis [23,24]. Damaged grains: As compared to seeds with intact husks, damaged seeds are more susceptible to AFs infection, according to numerous studies. An infestation of insects, poor food processing and inappropriate harvesting techniques can all harm the seed husk [25].
Overview of the global occurrence of AFs in grains
Many reports about the occurrence of AFs in food and products are available, especially with the advancement of analytical instruments and techniques. Nevertheless, at this point in this review, I focus on the natural contamination of AFs in only three types of grains, including corn, wheat, and rice, in major grainproducing countries, as well as in Egypt. AFs contamination of grains and products based on grains affects trade and the economy in both developed and developing countries. In the United States, corn producers lose $160 million a year because of contamination by AFs [26]. According to [27], these numbers are higher in developing nations, particularly in sub-Saharan Africa, where losses total $450 million. Some grains often contain more than one type of AFs. According to [28], 18304 samples of corn, (15889) wheat (2210), and rice (205) were collected from 100 countries during January 2008–December 2017. They found that AFB1 was detected in 24%, 10%, and 31% of the samples, respectively. In addition, 41.1%, 38.5%, and 20.9% of samples of corn from South Asia, Sub-Saharan Africa, and Southeast Asia, respectively, exceeded the maximum level for AFB1 (20 μg/kg). [29] reported that of the 41 and 25 studies surveyed on the occurrence of AFs in corn and wheat from 2018 to 2020, they concluded that the results of this literature review showed AFB1 was detected in 87.5 and 40% of corn and wheat samples, respectively.
A review study by [30] concluded that the data from around 17149 analyses by the European Food Safety Authority and data released from recent large surveys on aflatoxins occurrence across the world by Biomin suggest that aflatoxins prevalence is highest in Asia (25%), Europe (7%), the Middle East and North Africa (7%), and South and Central America (19%), as shown in (Table 1). Concerning AFB1, it is one of the most widespread aflatoxins commonly found in cases of aflatoxicosis. Also, it has a specific clause within the permissible limits. Therefore, (Table 2) clarifies the occurrence of AFB1 in the different regions of the world. Chandravarnan et al. (2024) in their systematic review of the prevalence of mycotoxins in rice from 2890 studies conducted from 2000 to 2023 showed that total aflatoxins ranked first (56%), while AFB1 recorded the third rank (34%), for the highest prevalence of mycotoxins in rice. On the other hand, concentration AFB1 (56.17 μg/kg).


Monitoring the presence of AFs during food (including grain) trade between countries
In this section, I draw on a collection of sources that offer a worldwide overview of aflatoxins’ distribution between 2018 and 2023. These sources include data from international organizations that monitor food safety globally, including the Joint Expert Committee on Food Additives and Contaminants (JECFA) and European Union (EU) Rapid Alert System for Food and Feed (RASFF). as well as certain recent systematic reviews. Statistics from [31] show that 400 cases of mycotoxin were reported as hazards; AFs only accounted for 367 (91.7%) alerts, which is approximately 10% of the total RASFF notifications this year [31].
[32] conducted a comprehensive analysis of risk assessments of aflatoxins published between 2016 and 2022. Based on the EU’s Rapid Alert System for Food and Feed (RASFF) data, they came to the conclusion that grains and their products represent the fourth most significant food category contaminated by AFs (3%) from these notifications. Corn and rice have been found to be the main sources of dietary intake from grains for individuals with AF at 2.19% and 0.71%, respectively. It comes in seventh and tenth rank per food category. In this regard, [33] reported that AFs are present in 60–80% of the world’s grain harvests. As well, for RASFF data, 2812 AFs notifications were found in different countries issued within the same period (January 2016 and March 2022), as shown in (Figure 2).
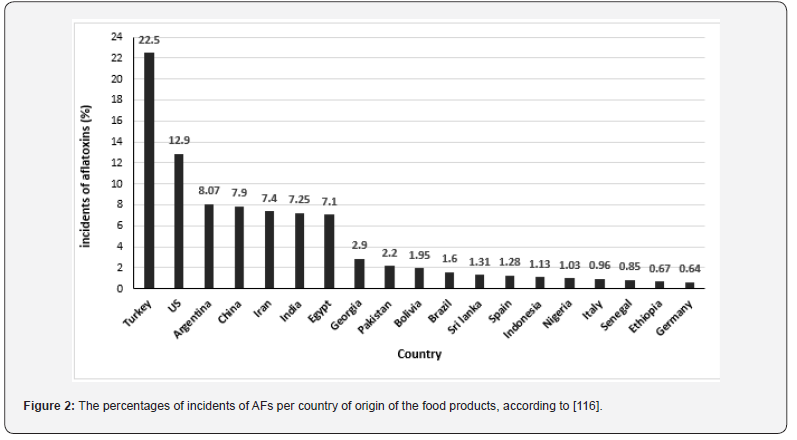
According to the data from [34] on the World Mycotoxin Survey, which was conducted on 21287samples from 86 countries, overall, the survey shows that the occurrence of fumonisins and deoxynivalenol remains high on every continent. Although the prevalence of mycotoxins is shifting, “due to climate change, mycotoxins, which were usually found in the southern part of the world, are now moving to the north. Mycotoxins are moving with the shifting climate,” said Annelies Mueller, product manager at Biomin. (Figure 3) shown the rate of the prevalence of AFs in some geographical areas in world.
The global production of main grains
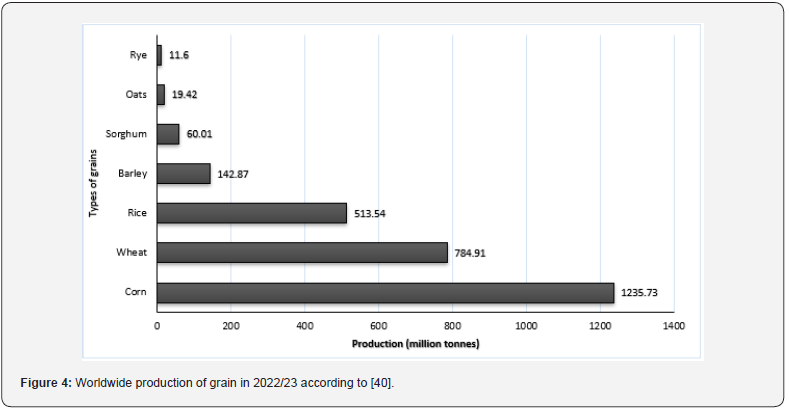
The production of grains increased by 64 million tonnes, or 2.1%, globally between 2020 and 2021, mostly because of a 4.1% increase in corn. Corn, wheat, and rice accounted for 90% of the total amount of grains produced in 2021. Corn, wheat, rice, barley, and sorghum are the five most produced species of grains (Figure 4). Corn showed the highest production (1.235 billion metric tons in 2023), followed by wheat (784.91 million metric tons) and rice (513.55 million metric tons). Concerning Egypt, according to CAPMAS (2022), the amount of production of corn, wheat, and rice was 7.2, 9.8, and 4.4 million metric tons, respectively. On the other hand, sorghum and barley recorded 750 and 90 metric tons, respectively [35]. The global production of corn produced by the United States and Brazil was 39%. Almost 23 percent of production was from China, making it the second-largest producer. Asia ranked first in the world for rice production, with the top three producers following China, India, and Bangladesh with percentages of 27, 25, and 7%, respectively. The total production of wheat in the world is centered in China (18% of the world total) and India (14%). The Russian Federation was the third-largest wheat producer, accounting for 10% of global production.

Natural occurrences of AFs in Egypt and the biggest producers of corn grains
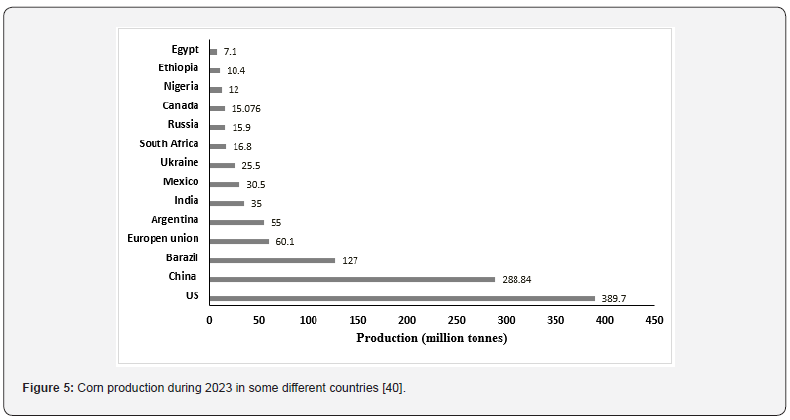
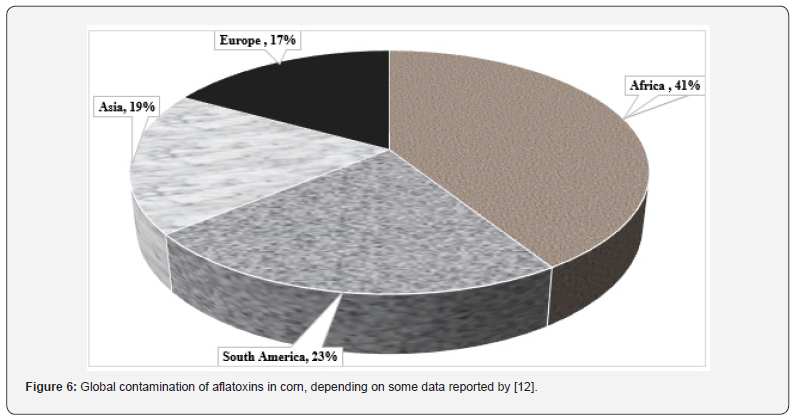
Corn is one of the most important crops in the world that are currently stored for animal feed and human consumption. According to the (IGC) International [36], the leading exporters of corn in the world are the US, Brazil, and Argentina by 369, 113, and 57 million tons, respectively, which are main producers of corn [36]. On the other hand, (Figure 5) displays the global corn production in 2023 as reported by [35]. The occurrence of AFs in corn can start before harvest, during harvest, and postharvest during storage when conditions are favorable for aflatoxigenic fungi for the growth and production of AFs [33]. AFs levels in corn grain vary from year to year but are typically highest in heatand drought-stressed years [37]. Consequently, AFs production may also alter periodically and significantly at a given location. According to [38], hot and dry weather patterns with drought episodes proved favorable for higher AFB1 contamination. (Figure 6) shows the percentage of AFs contamination of corn on the world’s continents. Over the past five years, numerous investigations have detected AFs in corn and its derivatives. According to data published by [12], more than 76% of total AFs were detected, followed by 20% of AFB1 and the remaining AFB2. The concentrations for total AFs ranged between 0.01 and 3760 μg/kg and AFB1 0.15–2072 μg/kg. Many studies have documented the contamination of corn by AFs. (Table 3) in this review displays the total AFs and AFB1 natural occurrences in samples from Egypt, as well as the biggest country produced for corn.

Natural occurrences of AFs in Egypt and the biggest producers of wheat grains
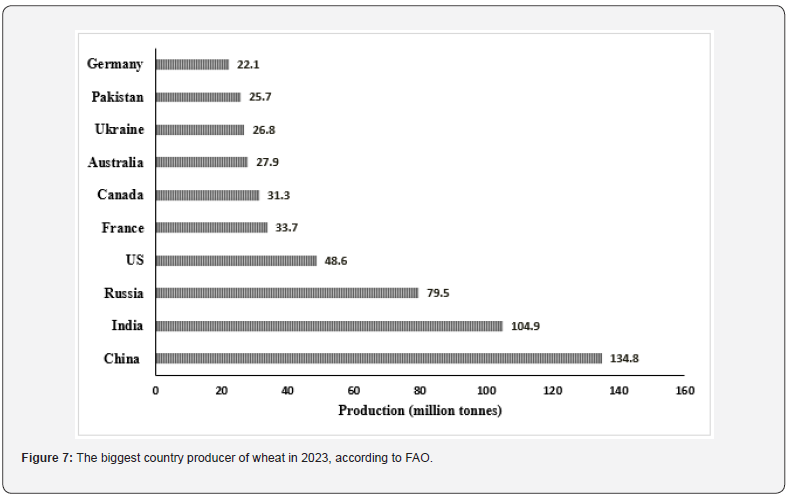
Wheat is an economic and important grain that supplies a fifth of global food calories and protein. It is one of the main staple foods in developing countries. Egypt has one of the highest wheat per capita consumption levels in the world (156.1 kg/person/ year). Egypt is eighth in the world in wheat consumption at a rate of 20.6 million tons annually, while the total production of wheat in Egypt was 9.1 million tons harvested from 3.2 million Feddan [39,40]. On the other hand, the top 10 wheat-producing countries in the world are display in (Figure 7). As shown in (Table 4), a number of studies have documented the presence of AFs in wheat. [28] reports that AFB1 found in 23% of 74,821 samples of wheat collected from 100 different countries worldwide and that the average concentration of infected samples was rising in samples taken from East Asia.

Natural occurrences of AFs in Egypt and the biggest producers of rice grains
Asia is the primary region for the cultivation and consumption of rice. Rice ranks third in the world after corn and wheat. As (Figure 8) illustrates, China and India are the world’s top producers. In Africa, rice is mainly produced in Egypt and Nigeria. Fungi that produce AFs can contaminate rice during harvest, handling, and storage, as well as when the field’s climate becomes conducive to their growth. Several studies have reported the presence of AFs in rice, which are highly prevalent in Asian nations. The significant frequency of AFs contamination in rice and rice-derived products highlights the significance of close observation of this staple food around the globe [41,42]. AFs prevalence in rice worldwide is displayed in (Table 5). According to [12], the number of AFs present in rice during the 2015–2020 period (with concentrations ranging from 0.014 to 921.93 μg/kg) accounts for 65% of the total aflatoxins. AFB1 and AFB2 make up 35%, with concentrations between 0.014 and 44.10 μg/kg.


Regulatory limits and standards of aflatoxins in grains
Depending on the potential human health risks posed by the dietary intake of AFs that have been assessed by several scientific bodies, such as FAO/WHO, JECFA, and EFSA. Many countries and international organizations have developed regulatory limits for the presence of AFs in food, including grain (cereals). In Egypt, the regulatory limits as recommended by EOSQC are compliant with EC No. 1881/2006, as shown in (Table 6).

EC: European Commission; FDA: Food and Drug Administration.
FSSAI: Food Safety and Standards Authority of India; FAMIC: Food and Agricultural Materials Inspection.
Centre; EOSQC: Egyptian Organization for standardization and Quality Control.
Post-harvest management to mitigate aflatoxins in grain
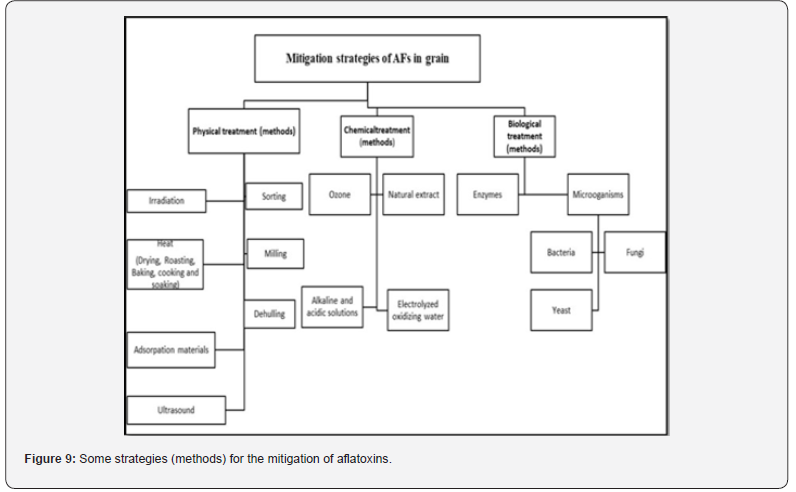
AFs can be found in all stages of grain production, from preharvest to processing, going through a harvest, and post-harvest stages; each stage has appropriate methods and strategies to mitigate and/or remove AFs. In the stage of pre-harvest, methods for controlling aflatoxins are largely preventive, like farming resistant varieties, reducing plant stress, proper fertilization, insect and weed control with the use of fungicides, and timely harvesting, as well as avoiding mechanical damage to the grain and rapid drying. However, this part will focus on the methods and strategies applied in the post-harvest stage, which includes the storage stage too. Strategies for mitigating or controlling aflatoxin in cereals are a viable means of ensuring that customers will receive safe and wholesome food because they prevent contamination of raw materials and processed goods [9]. (Figure 9) summarizes the various innovative strategies for the control of AFs in grain.
Any method or strategy used for the mitigation of AFs must meet a set of conditions, such as having no negative effect on either nutritional properties or food safety; not changing the physicalchemical properties of the treated foods significantly; and not leaving any toxic residues of the aflatoxins in the food products. In addition, its impact on AFs is an irreversible change; it is it is an environmentally friendly method. Lastly, these methods have easy-to-use, cost-effective, and safe post-harvest tools during storage and food processing [43-45]. Although the prevention of aflatoxins contamination in the field is the main goal of the agricultural and food industries, under certain environmental conditions, the contamination of several commodities with fungi produced for AFs may be unavoidable for producers. Once fungi or contamination with aflatoxins infects grains, different strategies and treatment options are available to improve the quality of the contaminated grains, which include chemical, biological, and physical treatments (Park, 2000). Irradiation, including gamma, ultraviolet (UV), and electron beams, for contaminated grain is considered an effective method for degradation of aflatoxins based on some factors like the UV dose and treatment time.
Post-harvest mitigation of AFs using physical methods or techniques
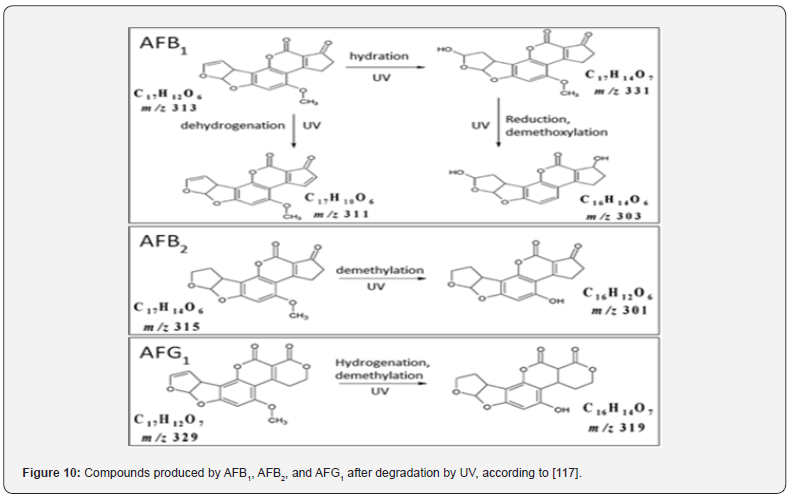
Numerous studies have revealed that physical strategies implemented after harvest are effective in reducing AF levels. For example, [46] reported that hand sorting by visible fungi infection is very efficient to decrease the AFB1 concentration of corn. Nevertheless, this approach is only applicable on an industrial scale using optical sorting equipment. Hulling of corn removes more than 90% of the AFs content, and rice polishing is a recommendable process [47,48]. The degradation of AFs requires high temperatures ranging from 237 to 306°C. According to reports, to achieve partial elimination of the toxins, temperatures must be above 150°C. Most industrial processes do not detoxify AFs, so there must be detection of AFs in final products. However, rice contaminated with AFB1 after cooking has shown a reduction of 34% that increased to 88% with pressure-cooking [49]. Sorting of grains leads to a decrease in AFs levels as clean grains are physically separated from contaminated ones. Nevertheless, this method is not very practical due to the incomplete removal of AFstainted grains Schaarschmidt and [50]. Milling of contaminated grain with AFs leads to the redistribution of toxin in certain mill fractions without destroying it [51]. Irradiation, including gamma, ultraviolet (UV), and electron beams, for contaminated grain is considered an effective method for the degradation of aflatoxins based on some factors like the dose and treatment time. According to [52], gamma (γ) is the most preferred radiation source for treating food with doses up to 10 kGy. On the other hand, UV irradiation is highly cost-effective and eco-friendly. Treatment of grains with moderate doses has no negative impact on their sensory and physicochemical properties. Many studies showed that UV treatment was effective. Most studies reported that the sensitivity of aflatoxins to UV was AFB1 > AFG1 > AFB2 [53]. Treatment of AFs leads to the appearance of several degraded products, as shown in (Figure 10).
The application of ultrasound for the reduction or degradation of aflatoxins in grains is a promising technology because of its minimal impact on the physicochemical properties of foods. It also produces no secondary pollutants, so it is an eco-friendly and non-polluting technique. AFB1 degraded by ultrasound by affecting the chemically stable furan moiety and by changing the lactone ring in the main structure of AFB1 as shown (Figure 11 A &B) [54,55].

Post-harvest mitigation of AFs using chemical methods or techniques
Numerous chemicals have been tested for their ability to degrade or detoxify aflatoxins, including acids, ammonia (ammoniation), natural extracts from plants, and ozone gas (ozonation). Although many of the proposed treatments may successfully destroy aflatoxins, however, after many studies, it became clear that many of them are not suitable for application due to their many problems, such as sensory and nutritional properties [56]. In addition, the ammoniation process generates toxic products [57]. On the contrary, the ozonation process is one of the most important methods in this field and the most suitable for application with grains at all stages of production [58,59]. In this point, will shed light on some of these methods, their results, and their working mechanisms.
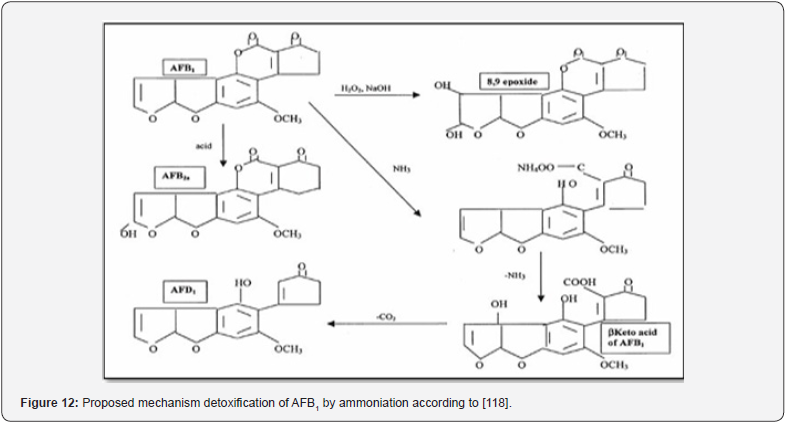
Ammoniation was one of the degradation methods for aflatoxins in the past, which used NH4OH or NH3. It can be utilized in two ways: as a high-pressure and high-temperature process or as an atmospheric pressure and ambient temperature process. (Figure 12) shows that this process begins with the opening of the lactone ring of AFB1 (reversible) and followed by ammonium salt forming from the resulting hydroxy acid. According to [60], using higher ammonia concentrations resulted in an increase in the ammoniation process’s efficiency. Organic acids have been used for AFs degradation through the soaking process of grains, for instance, soaking of grain in 1.0 N tartaric acid, lactic acid, and citric acid for 18 h at room temperature, which leads to a degradation of the AFB1 level by 95.1, 92.7, and 94.1%, respectively [61]. To get the best results, it is preferable to combine them with other mitigation technologies (Rastegar et al., 2017). The use of organic acid for the treatment-contaminated grains is expensive [62].
Some authors have previously reported on the use of natural extracts and essential oils for the decrease or degradation of AFs, especially in vitro, in different ways, like inhibiting the growth of aflatoxigenic fungi, blocking AF biosynthesis, and removing or degrading AFs [63-65]. Because plant extracts contain a large group of active compounds, as shown in (Figure 13). The most important advantage of using plant extracts to control aflatoxin is that their effect on food quality is limited. However, most of them lack specific mechanisms of action. Therefore, it needs more research to benefit from it on a commercial scale [66]. In this regard, I have conducted a study using the aqueous extract of three leaves of wild edible plants (sow thistle, chicory, and Rajesh), which has proven effective in preventing the synthesis of AFs with percentages of inhibition of AFB1 of 78.03, 68.8, and 81.7%, respectively [67]. Another study by [68] found that using an aqueous extract of carob pulp at 5 mg/mL reduced the production of AFs by 76.5 to 86.5%.
Ozonation has been considered an interesting method for the remediation of cereals contaminated by aflatoxins. The ozone reaction with AFB1 in the site C8-C9 double bond at the terminal furan leads to the formation of aflatoxin molozonide, which is further changed to aflatoxin ozonide. This compound is unstable and changes to aldehydes, ketones, acids, and CO2 (Figure 14). Ozone decomposes to form oxygen gas and therefore can be classified as a nonpersistent chemical; however, it must be generated at the location of its intended use (McKenzie et al., 1997). On the other hand, these features make ozone an important alternative for the food industry. Finally, many international organizations, including the WHO, FAO), and FDA, had regarded ozone as a safe and effective chemical applied in the food industry. A few factore, including temperature, moisture content, exposure time, and O3 concentration, which effected on effectiveness of ozonation process on aflatoxins. A few factors, including temperature, moisture content, exposure time, and O3 concentration, affect the degradation of aflatoxins by ozone [69,70]. In my opinion, it is one of the most effective ways to reduce aflatoxins. It also has ease of application in gaseous as well as liquid forms, no residue after contact, no hazardous disposal, and easy on-site generation of ozone [71].
Post-harvest mitigation of AFs using biological methods or techniques
Biological methods for degradation or mitigation of AFs involve microorganisms (fungi, bacteria, and yeast) or enzymes that lead to less toxic or non-toxic metabolites. This method has emerged as an efficient and eco-friendly strategy for the degradation of AFs. Due to its safety, sustainability, and economic viability, the biocontrol method offers an appealing and suitable substitute for the removal and degradation of AFB1 from food [72]. However, it has some drawbacks, such as the difficulty of controlling microbial performance, the safety of the newly formed product to the body, and some of them are only active in certain environmental conditions [73].
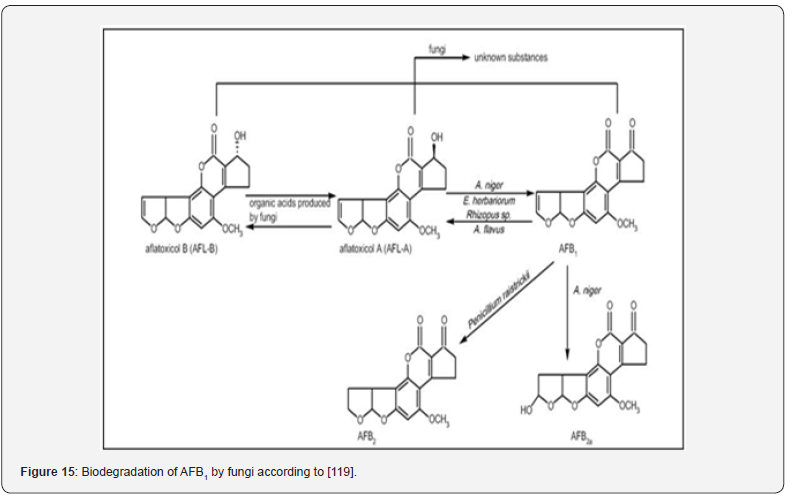
There are many antagonistic microbes that can be used for this purpose, such as several bacterial species, nontoxigenic fungi, and trichoderma. The biological techniques are based on competitive exclusivity or biological interactions like antibiosis. Biodegradation for AFB1 may work through catabolic pathways to detoxify the AFs into less toxic intermediates or final products. Such as, microbe is able to convert AFB1 to aflatoxicol by reducing the cyclopentenone carbonyl of AFB1. These fungi could convert AFB1 to aflatoxicol-A, and then aflatoxicol-A was converted to aflatoxicol-B by the actions of medium components or organic acids produced from the fungi (Figure 15).
Lactic acid bacteria (LAB) such as Lactobacillus, Bifidobacterium, and Propionibacterium are the most studied of all bacteria used for the biodegradation of AFs. LAB has demonstrated a great potential for removing AFs and can be utilized as starter cultures in the fermentation of foods and as additives in food processing. The mechanism of reducing AFs by LAB is due to their adhesion to cell-wall components [74]. As well, LAB produces many active compounds that change the original structure of the AFS and convert them into substances that are low in toxicity or even completely non-toxic [8]. Lactic acid bacteria are a promising strategy to mitigate aflatoxins contamination of grains. Especially if it is used in liquid media like fermentation processes for some grains, such as barley and other grains [75]. In this field, I have conducted research with a group of researchers, such as Fouad and [76], they evaluated three strains to reduce aflatoxins in wheat grains and found that the highest inhibition rates of AFs ranged between 61.4 and 75.8% with Lactobacillus rhamnosus [76]. In another study of ours, we evaluated fourteen strains of LAB isolated from dairy products to reduce AFs. We found that all tested strains were able to reduce AFB1 at different rates depending on the time of incubation. Increasing the incubation time to 36 hours led to removing more than 88 from AFB1 [77]. As well as non-toxic fungi can be used. [78] used the fungus Trichoderma harzianum to biodegrade AFB1. They found that the greatest degradation was 76.8%, which led to a 65% suppression of A. flavus growth.


Recently, many reports on the isolation, identification, and purification of AFs-degrading enzymes from microorganisms have increased significantly. To avoid defects resulting from the use of whole organisms for biodegradation for AFs, the use of enzymes is far more convenient since they are substrate-specific, effective, and environmentally friendly; moreover, their application in the food and feed industries has been established [79]. The enzymatic degradation of AFs depends on a number of factors, including temperature, incubation time, enzyme concentration, and initial AFs concentration [80]. Alberts et al. (2009) first proposed the role of laccases that produced and purified from Pleurotus pulmonarius in degradation of AFB1. They reported that enzyme laccase (Lac2) showed AFB1 degradation up to 90%. According to [81] laccases act on AFB1 in two ways as shown in (Figure 16).

Controlling and mitigation strategies for aflatoxins during storage grains Sorting and cleaning
The most important thing about grain storage is that AFs levels will not increase if the grain is properly stored but may increase if it is not. Thus, the lower the level of AFs in the grain when it is stored, the lower the levels in the grain when it is taken out of storage. Although it would be better if the grains were free of AFs from the beginning, on the other hand, it is difficult for grains to be free of fungi and their spores, which develop under storage conditions and produce AFs. Therefore, it is extremely important to reduce the microbial load of stored grains before storage by any methods such as ozonation or washing. AFs contamination during storage can be greatly reduced by using a combination of cleaning techniques to effectively remove grain that is clearly moldy, sick, broken, and/or damaged. According to [82] and Schaarschmidt and [50], cleaning grains leads to removing 7–50% of the toxin contaminating the grain. AFs were shown to be reduced by 40–80% when damaged and contaminated corn grains were removed. Furthermore, sifting broken and damaged grains by hand eliminated 95% of AFs. The initial concentrations in grains and the percentage of pollutants removed throughout the cleaning process determine how much the cleaning process can reduce aflatoxin levels Park, 2000 [46].
Storage conditions and management techniques
The temperature, grain moisture content, and relative humidity during storage are the main factors that must be under control. As well, which must be managed efficiently and professionally.
A moisture content
10–14% is ideal for drying grain. Since grain is often dry when harvested, it is allowed to put it in storage if the aw is less than 0.70 [83,84]. As well, proper monitoring of temperature and relative humidity.
Regulate the temperature
At low or cold temperatures, fungal is not killed, but growth will be slow, and metabolism (production AFs) is more difficult to occur at lower temperatures. Keeping the grain piles at a consistent temperature and practicing proper hygiene are sufficient and essential storage precautions [48].
Control of insects
It is necessary to manage the presence of insects since the majority of insects in storage systems have the ability to promote the growth of fungus by increasing the temperature of the grains and moisture, all of which promote the production of AFs [85,86].
Modification of atmosphere during storage
Changes to the atmospheric gases, such as CO2 and N2, in storage silos could stop or at least lessen the generation of AFs. Certain aflatoxins have been shown to be inhibited and fungal growth on grain to be prevented by <1% from O2 and/or increasing CO2 or N2 concentrations [87, 120-124].
Future vision for the development and sustainability of controlling methods of AFs
In this section, I highlight a few points that I think merit more
investigation in the future.
Designing a program to monitor and predict aflatoxins
during grain storage
Using artificial intelligence (AI) systems to combine
more than one method of reducing aflatoxin toxins.
Relying on sustainable methods to combat or reduce the
presence of aflatoxins
Use the equipped robots to monitor storage operations
inside the silos.
Developing packaging materials using nanotechnology
to prevent the growth of fungi and the production of their toxins
on grains
Developing detection and analysis methods in order to
reduce costs (sampling, analysis, and storage) during monitoring
storage.
The transformation pathways and transformation
products of aflatoxins still require more research.
We still need to work on developing some technologies
to make it safer, more environmentally friendly, and faster.
- Abdel-Wahhab MA, El-Nekeety AA, Aly SA (2019) Mycotoxins in children problem and halal management. Int J Halal Res 1(1): 16-38.
- Frisvad JC, Hubka V, Ezekiel CN, Hong SB, Nováková A, et al. (2019) Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud Mycol 93: 1-63.
- Pandey MK, Kumar R, Pandey AK, Soni P, Gangurde SS, et al. (2019) Mitigating Aflatoxin Contamination in Groundnut through A Combination of Genetic Resistance and Post-Harvest Management Practices. Toxins 11(6): 1-21.
- Abdel-Wahhab MA, El-Nekeety AA, Hassan NS, Gibriel AAY, Abdel-Wahhab KG (2018) Encapsulation of cinnamon essential oil in whey protein enhances the protective effect against single or combined sub-chronic toxicity of fumonisin B1 and/or aflatoxin B1 in rats. Environ Sci Pollut Res Int 25(29): 29144-29161.
- Abdel-Wahhab MA, Salman AS, Ibrahim MIM, El-Kady AA, Abdel-Aziem SH, et al. (2016) Curcumin nanoparticles loaded hydrogels protects against aflatoxin B1-induced genotoxicity in rat liver. Food Chem Toxicol 94: 159-171.
- Khaneghah MA, Es I, Raeisi S, Fakhri Y (2018) Aflatoxins in cereals: State of the Art. J Food Saf 38(6): e12532.
- Wan J, Chen B, Rao J (2020) Occurrence and Preventive Strategies to Control Mycotoxins in Cereal-based Food. Compr Rev Food Sci Food Saf 19(3): 928-953.
- Guan Y, Chen J, Nepovimova E, Long M, Wu W, et al. (2021) Aflatoxin detoxification using microorganisms and enzymes. Toxins 13(1): 46.
- Sirhan AY, Tan GH, Al Shunnaq A, Abdulrauf L, Wong RC (2014) QuEChERS-HPLC method for aflatoxin detection of domestic and imported food in Jordan. J Liq Chromatogr Relat Technol 37: 321-342.
- Nazhand A, Durazzo A, Lucarini M, Souto EB, Santini A (2020) Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 9(5): 1-26.
- Bumbangi NF, Muma JB, Choongo K, Mukanga M, Velu MR, et al. (2016) Occurrence and factors associated with aflatoxin contamination of raw peanuts from Lusaka district's markets, Zambia. Food Control 68: 291-296.
- Gómez‐Salazar JA, Ruiz‐Hernández K, Martínez‐Miranda MM, Castro‐Ríos K (2021) Postharvest strategies for decontamination of aflatoxins in cereals. Food Rev Int 39(7): 1-
- Lv C, Jin J, Wang P, Dai X, Liu Y, et al. (2019) Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem 293: 472-478.
- Gasperini AM, Medina A, Magan N (2022) Comparison of growth and aflatoxin B1 production profiles of Aspergillus flavus strains on conventional and isogenic GM-maize-based nutritional matrices. Fungal Biol 126(1): 82-90.
- Schmidt Heydt M, Rüfer CE, Abdel Hadi A, Magan N, Geisen R (2010) The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to afl R expression. Mycotoxin Res 26(4): 241-246.
- Ding N, Xing F, Liu X, Selvaraj JN, Wang L, et al. (2015) Variation in the fungal microbiome (mycobiome) and aflatoxin in stored in-shell peanuts at four different areas of China. Front Microbial 6: 1055.
- Mannaa M, Kim KD (2017) Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Microbiology 45(4): 240-254.
- Eshelli M, Harvey L, Edrada-Ebel R, McNeil B (2015) Metabolomics of the bio-degradation process of aflatoxin B1 by actinomycetes at an initial pH of 6.0. Toxins 7(2): 439-56.
- Rushing BR, Selim MI (2019) Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 124: 81-100.
- Priesterjahn EM, Geisen R, Schmidt Heydt M (2020) Influence of light and water activity on growth and mycotoxin formation of selected isolates of Aspergillus flavus and Aspergillus parasiticus. Microorganisms 8(12): 1-15.
- Bowen Tai, Jinghua Chang, Yang Liu, Fuguo Xing (2020) Recent progress of the effect of environmental factors on Aspergillus flavus growth and aflatoxins production on foods. Food Quality and Safety 4(1): 21-28.
- Liu J, Sun L, Zhang N, Zhang J, Guo J, et al. (2016) Effects of nutrients in substrates of different grains on aflatoxin B1 production by Aspergillus flavus. Biomed Res Int. 2016: 7232858.
- Chang PK, Hua SST, Sarreal SBL, Li RW (2015) Suppression of aflatoxin biosynthesis in Aspergillus flavus by 2-phenyl ethanol is associated with stimulated growth and decreased degradation of branched-chain amino acids. Toxins 7(10): 3887-3902.
- Majumdar R, Minocha R, Lebar MD, Rajasekaran K, Long S, et al. (2019) Contribution of maize polyamine and amino acid metabolism toward resistance against Aspergillus flavus infection and aflatoxin production. Front Plant Sci 10(692): 1-16.
- Lahouar A, Marin S, Crespo-Sempere A, Saïd S, Sanchis V (2016) Effects of temperature, water activity and incubation time on fungal growth and aflatoxin B1 production by toxinogenic Aspergillus flavus isolates on sorghum seeds. Rev Argent Microbiol 48(1): 78-85
- Wu F (2015) Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J 8(2): 137-142.
- Gbashi S, Madala NE, De Saeger S, De Boevre M, Adekoya I, et al. (2018) The Socio-economic impact of mycotoxin contamination in Africa [Internet]. Mycotoxins - impact and management strategies Intech Open 2019.
- Gruber-Dorninger C, Jenkins T, Schatzmayr G (2019) Global mycotoxin occurrence in feed: A Ten-Year Survey. Toxins 27 11(7): 375.
- Khodaei D, Javanmardi F, Khaneghah AM (2020) The global overview of the occurrence of mycotoxins in Cereals: A three-year survey. Curr Opin Food Sci 39: 36-42.
- Yu J, Pedroso IR (2023) Mycotoxins in cereal-based products and their impacts on the health of humans, livestock animals and pets. Toxins 15(8): 1-41.
- EC (European Commission). (2021) RASFF Annual report 2020.
- Bhardwaj K, Meneely JP, Haughey SA, Dean M, Wall P, et al. (2023) Risk assessments for the dietary intake aflatoxins in food: A systematic review (2016-2022). Food Control 149: 109687.
- Eskola M, Kos G, Elliott CT, Hajšlová J, Mayar S, et al. (2020) Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25%. Crit Rev Food Sci Nutr 60(16): 2773-2789.
- Biomin (2020) Biomin World Mycotoxin Survey 2020. Annual Report No Pp: 17.
- FAOSTAT (Statistics at Food and Agriculture Organization of the United Nations) (2023): World maize wheat rice soybeans potatoes and cotton production.
- IGC (2021) (International Grains Council) Five-year Baseline Projections of Supply and Demand for Wheat, Maize (Corn), Rice and Soyabeans to 2025/26 International Grains Council P: 8.
- Damianidis D, Ortiz BV, Bowen KL, Windham GL, Hoogenboom G, et al. (2018) Minimum temperature, rainfall, and agronomic management impacts on corn grain aflatoxin contamination. J Agron 110(5): 1697-1708.
- Dövényi-Nagy T, Rácz C, Molnár K, Bakó K, Szláma Z, et al. (2020) Pre-Harvest modelling and mitigation of aflatoxins in maize in a changing climatic environment A Review. Toxins 12(12): 768.
- (2022) Central Agency for Public Mobilization and Statistics (CAPMAS). Central Agency for Public Mobilization and Statistic. Cairo Egypt.
- FAO (2023) Agricultural production statistics. 2022-2023. FAOSTAT Analytical Brief Series No. 60. Rome.
- Sun XD, Su P, H Shan (2017) Mycotoxin contamination of rice in China: mycotoxin contamination of rice in China. J Food Sci 82(3): 573-584.
- Ali N (2019) Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol Rep 6: 1188-1197.
- Chulze SN, Palazzini JM, Torres AM, Barros G, Ponsone ML, et al. (2015) Biological control as a strategy to reduce the impact of mycotoxins in peanuts, grapes and cereals in Argentina. Food Addit Contam Part A 32(4): 471-479.
- Lagogianni CS, Tsitsigiannis DI (2019) Effective biopesticides and bio stimulants to reduce aflatoxins in maize fields. Front Microbiol 10: 2645.
- Sipos P, Peles F, Brasso DL, Béri B, Pusztahelyi T, et al. (2021) Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 13(3): 204.
- Matumba L, Van Poucke C, Ediage EN, Jacobs B, De Saeger S (2015) Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin-contaminated white maize. Food Addit Contam 32(6): 960-969.
- Castells M, Ramos AJ, Sanchis V, Marín S (2007) Distribution of total aflatoxins in milled fractions of hulled rice. J Agric Food Chem 55(7): 2760-2764.
- Peng WX, Marchal JLM, Van der Poel AFB (2018) Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim Feed Sci Tech 237: 129-153.
- Park JW, Kim Y B (2006) Effect of pressure-cooking on aflatoxin B1 in rice. J Agric Food Chem 54(6): 2431-2435.
- Schaarschmidt S, Fauhl Hassek C (2018) The fate of mycotoxins during the processing of wheat for human consumption. Comp Rev Food Sci Food Saf 17(3): 556-593.
- Cheli F, Pinotti L, Rossi L, Dell’ Orto V (2013) Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT-Food Sci and Technol 54(2): 307-314.
- WHO (1999) High-dose irradiation: wholesomeness of food irradiated with doses above 10 kGy. Report of a joint FAO/IAEA/WHO study group. World Health Organization technical report series 890: 1-197.
- Mao J, He B, Zhang L, Li P, Zhang Q, et al. (2016) A structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV Irradiation. Toxins 8(11): 1-11.
- Liu Y, Li M, Liu Y, Bian K (2019) Structures of reaction products and degradation pathways of aflatoxin B1 by ultrasound treatment. Toxins (Basel) 11(9): 526.
- Liu Y, Liu Y, Zhao W, Li M, Liu N, et al. (2022) Reduction of aflatoxin B1 and zearalenone contents in corn using power ultrasound and its effects on corn quality. Toxins 30 14(12): 834.
- Park DL (1997) Perspectives on mycotoxin decontamination procedures. Food additives and contaminants 10(1): 49-60.
- Hernandez AB, Price RL, Kornman K, Garcia R, Njapau H, et al. (2002) Decontamination of aflatoxin B1 contaminated corn by ammoniation persulphate during fermentation. Sci Food Agric 82: 546-552.
- El-Desouky TA, Sharoba AMA, El-Desouky AI, El-Mansy HA Khayria Naguib (2012 a) Effect of ozone gas on degradation of aflatoxin B1 and Aspergillus flavus fungal. J Environ Anal Toxicol 2(2): 1000128.
- Sivaranjani S, Prasath VA, Pandiselvam R, Kothakota A, Khaneghah AM (2021) Recent advances in applications of ozone in the cereal industry. LWT 146: 111-412.
- Gomaa MNE, Ayesh AM, Abdel Galil MM, Naguib K (1997) Effect of high pressure ammoniation procedure on the detoxification of aflatoxins. Mycotoxin Res 13(1): 23-34.
- Lee J, Her JY, Lee KG (2015) Reduction of aflatoxins (B₁, B₂, G ₁, and G₂) in soybean-based model systems. Food Chem 189: 45-51.
- Abuagela MO, Iqdiam BM, Mostafa H, Marshall SM, Yagiz Y, et al. (2019) Combined effects of citric acid and pulsed light treatments to degrade B-aflatoxins in peanut. Food Bioprod Process 117: 396-403.
- Vijayanandraj S, Brinda R, Kannan K, Adhithya R, Vinothini S (2014) Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica nees. Microbiol Res 169(4), 294-300.
- Tian F, Chun HS (2017) Natural Products for Preventing and Controlling Aflatoxin Contamination of Food. Intech London.
- Ahmad MM, Qamar F, Saifi M, Abdin MZ (2022) Natural inhibitors: A sustainable way to combat aflatoxins. Front Microbiol 13: 993834.
- Negera M, Washe AP (2019) Use of natural dietary spices for reclamation of food quality impairment by aflatoxin. J Food Qual 4: 1-10.
- El-Desouky TA (2021) Evaluation of effectiveness aqueous extract for some leaves of wild edible plants in Egypt as anti-fungal and anti-toxigenic. Heliyon 7(2): e06209.
- El-Desouky TA (2022) Protect peanut kernels from Aspergillus spp and their mycotoxins during storage by aqueous extract of carob pulp. Discov Food 2: 25.
- El-Desouky TA, Sharoba AMA, AI El-Desouky, El-Mansy HA, Khayria Naguib (2012 b) Evaluation of ozone gas as an anti-aflatoxin B1 in wheat grains during storage. Journal of Agroa Proc and Tech 18(1): 13-19.
- Trombete FM, Porto YD, Freitas Silva O, Pereira RV, Direito GM, et al. (2017) Efficacy of ozone treatment on mycotoxins and fungal reduction in artificially contaminated soft wheat grains. J Food Process Preserv 41(3): e12927.
- Pandiselvam R, Subhashini S, Banuu Priya EP, Kothakota A, Ramesh SV, et al. (2019) Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Science & Engineering 41(1): 17-34.
- Okwara PC, Afolabi IS, Ahuekwe EF (2021) Application of laccase in aflatoxin B1 degradation: A review. IOP Conf Ser Mater Sci Eng 11(7): 1-12.
- Adebo OA, Njobeh PB, Gbashi S, Nwinyi OC, Mavumengwana V (2017) Review on microbial degradation of aflatoxins. Crit Rev Food Sci Nutr 57(15): 3208-3217.
- Shetty PH, Jespersen L (2006) Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol 17: 48-55.
- Asurmendi P, Gerbaldo G, Pascual L, Barberis L (2020) Lactic acid bacteria with promising AFB1 binding properties as an alternative strategy to mitigate contamination on brewers' grains. J Environ Sci Health B 55(11): 1002-1008.
- Fouad MT, El-Desouky TA (2020) Anti-toxigenic effect of lactic acid bacteria against aspergillus spp isolated from wheat grains. The Open Microbiology Journal 14: 252-259.
- Fouad MT, El-Shenawy M, El-Desouky TA (2021) Efficiency of selected lactic acid bacteria isolated from some dairy products on aflatoxin B1 and ochratoxin A. J Pure Appl Microbiol 15(1): 312-319.
- Madbouly AK, Rashad YM, Ibrahim MIM, Elazab NT (2023) Biodegradation of aflatoxin B1 in maize grains and suppression of its biosynthesis-related genes using endophytic Trichoderma harzianum AYM3. J Fungi 9(2): 1-18.
- Schmidt MA, Mao Y, Opoku J, Mehl HL (2021) Enzymatic degradation is an effective means to reduce aflatoxin contamination in maize. BMC Biotechnol 21(1): 70.
- Branà MT, Sergio L, Haidukowski M, Logrieco AF Altomare C (2020) Degradation of aflatoxin B1 by a sustainable enzymatic extract from spent mushroom substrate of Pleurotus eryngii. Toxins 12(1): 49.
- Kumar V, Bahuguna A, Ramalingam S, Dhakal G, Shim JJ, et al. (2022) Recent technological advances in mechanism, toxicity, and food perspectives of enzyme-mediated aflatoxin degradation. Crit Rev Food Sci Nutr 62(20): 5395-5412.
- Tibola CS, Fernandes JMC, Guarienti EM (2016) Effect of cleaning, sorting and milling processes in wheat mycotoxin content. Food Cont 60: 174-179.
- Magan N, Aldred D (2006) Managing microbial spoilage in cereals and baking products. In Food Spoilage Microorganisms Pp: 194-212.
- Mrema G, Gumbe L, Chepete H, Agullo J (2011) Grain crop drying, handling and storage. In FAO Rural Structures in the Tropics: Design and Development; FAO: Rome Italy Pp: 363-383.
- Hell K, Cardwell KF, Setamou M, Schulthess F (2000) Influence of insect infestation on aflatoxin contamination of stored maize in four agroecological regions in Benin. African Entomology 8(2): 169-177.
- El-Desouky TA, Elbadawy SS, Hussain HB, Hassan NA (2018) Impact of insect densities Tribolium Castaneum on the benzoquinone secretions and aflatoxins levels in Wheat flour during storage periods. The Open Biotech Journal 12(1): 104-111.
- Sudini H, Rao GR, Gowda C, Chandrika R, Margam V, et al. (2015) Purdue Improved Crop Storage (PICS) bags for safe storage of groundnuts. J Stored Prod Res 64: 133-138.
- Biomin (2019) Biomin World Mycotoxin Survey 2020. Annual Report No Pp: 17.
- Abdallah M, Girgin G, Baydar T (2019) Mycotoxin detection in maize, commercial feed and raw dairy milk samples from Assiut City, Egypt. Vet Sci 6(2): 57.
- Sayed TI, El Desouky TA, Abd EAAM (2019) Investigation of fungus associated within co-occurrence of aflatoxins and ochratoxin A in cereals from Egypt. MOJ Toxicol 5(3): 92-
- Weaver AC, Weaver DM, Adams N, Yiannikouris A (2021) Co-occurrence of 35 mycotoxins: a seven-year survey of corn grain and corn silage in the United States. Toxins 13(8): 516.
- Hao W, Li A, Wang J, An G, Guan S (2022) Mycotoxin contamination of feeds and raw materials in china in year 2021. Front Vet Sci (9): 929904.
- Mallmann CA, Mallmann AO, Tyska D (2020) Survey of mycotoxin in Brazilian corn by NIR Spectroscopy-Year 2019. Glob J Nutri Food Sci 2(5): 1-7.
- Mudili V, Siddaih CN, Nagesh M, Garapati P, Naveen Kumar K, et al. (2014) Mould incidence and mycotoxin contamination in freshly harvested maize kernels originated from India. J Sci Food Agric 94: 2674-2683.
- Castellari CC, Cendoya MG, Marcos FV, Barrera V, Pacin AM (2015) Extrinsic and intrinsic factors associated with myco- toxigenic fungi populations of maize grains (Zea mays L.) stored in silo bags in Argentina. Revista Argentina de microbiologia 47(4): 350-359.
- Nji N, Christianah A, Njie A, Mulunda M (2022) Biodiversity and distribution of aspergillus and their toxins in maize from western and eastern regions of South Africa. Adv Microbio 12(3): 121-149.
- Ayeni KI, Akinyemi OM, Kovač T, Ezekiel CN (2020) Aflatoxin contamination of maize vended in Ondo state, Nigeria, and health risk assessments. Croat J food Sci Technol 12: 123-129.
- Kayola KK, Gebre SG, Addisu S, Amanuel DK (2024) Evaluation of aflatoxin content in “Cheka” traditional beverage in South-Western Ethiopia and its major ingredient (maize). Discov Food 4: 12.
- Yilma S, Sadessa K, Kebede D (2019) Infections F Aflatoxin Contamination in Maize Grains Collected from West Showa and East Wallega Zones, Ethiopia. Int J Curr Res Rev 11(21): 16-22.
- Hathout AS, Abel-Fattah SM, Abou-Sree YH, Fouzy AS (2020) Incidence and exposure assessment of aflatoxins and ochratoxin a in Egyptian wheat. Toxico Rep 7: 867-873.
- Elmaadawy AA, Eman B Mehram, Yousif A Elhassaneen (2019) Occurrence of aflatoxin B1 in wheat grains samples stored in Egyptian homes: seasonal and regional studies. Journal of the Faculty of Specific Education 22(5): 176-183.
- Zhao J, Cheng T, Xu W, Han X, Zhang J, et al. (2021) Natural co-occurrence of multi-mycotoxinsin unprocessed wheat grains from China. Food Cont 130(2): 108-321.
- Turksoy S, Kabak B (2020) Determination of aflatoxins and ochratoxin A in wheat from different regions of Turkey by HPLC with fluorescence detection. Acta Alimentaria 49(1): 118-124.
- Quiles JM, Saladin F, Mañes J, Fernández Franzón M, Meca G (2016) Occurrence of mycotoxins in refrigerated pizza dough and risk assessment of exposure for the Spanish population. Food Chem Toxicol 94: 19-24.
- El-Naggar A, Abdel‐Samie M, Ghoneim S (2018) Comparison of mycotoxins content four-imported wheat to Egypt. Sinai Journal of Applied Sciences 7(1): 59-70.
- Asghar MA, Ahmed A, Iqbal J, Zahir E, Nauman H (2016) Fungal Flora and Aflatoxin Contamination in Pakistani Wheat Kernels (Triticum Aestivum L.) And Their Attribution in Seed Germination. J Food Drug Anal 24(3): 635-643.
- Moharram A, Yasser M, Sayed M, Omar O, Idres M (2019) Microbiota and mycotoxins contaminating rice grains in El-Minia, Governorate, Egypt. Biosci Biotechnol Res Asia 16(1): 167-178.
- Fang L, Zhao B, Zhang R, Wu P, Zhao D, et al. (2022) Occurrence and exposure assessment of aflatoxins in Zhejiang province, China. Environ Toxicol Pharmacol 92:103847.
- Mukherjee A, Sharma M, Latkar SS (2019) A study on Aflatoxin content in black scented rice in India. Int J Pharm Anal Res 8(1): 125-130.
- Islam MZ (2021) Determination of aflatoxins in food grains and processed foods from selected areas in bangladesh with risk assessment. PhD thesis, Sher-e-Bangla Agricultural University Dhaka.
- Phan LTK, De Saeger S, Eekhout M (2023) Public health risk due to aflatoxin and fumonisin contamination in rice in the Mekong Delta, Vietnam. Food saf and Risk 10(4): 1-13.
- Sales AC, Yoshizawa T (2015) Updated profile of aflatoxin and Aspergillus section Flavi contamination in rice and its byproducts from the Philippines. Food Addit. Contam 22(5): 429-436.
- Xia L, Routledge MN, Rasheed H, Ismail A, Dong Y, et al. (2020) Biomonitoring of Aflatoxin B1 and Deoxynivalenol in a Rural Pakistan Population Using Ultra-Sensitive LC-MS/MS Method. Toxins 12(9): 591.
- Kpakpale DO, Kraak B, Meijer M, Ayeni KI, Houbraken J, et al. (2021) Fungal diversity and aflatoxins in maize and rice grains and cassava-based flour (Pupuru) from Ondo state, Nigeria. J Fungi 7(8): 635.
- Foodakai (2021) Eu Rasff alerts for aflatoxins from 04 January 2016. until 2nd March 2022.
- Patras A, Julakanti S, Yannam S, Bansode RR, Burns M, et al. (2017) Effect of UV irradiation on aflatoxin reduction: a cytotoxicity evaluation study using human hepatoma cell line. Mycotoxin Res 33(4): 343-350.
- Cucullu AF, Lee LS, Pons WA, Stanley JB (1976) Ammoniation of aflatoxin B1. Isolation and characterization of a product with molecular weight 206. J Agric Food Chem 24(2): 408-410.
- Wu Q, Jezkova A, Yuan Z, Pavilkove L, Dohnal V (2009) Biological degradation of aflatoxins. Drug Metab Rev 41(1): 1-7.
- EOSQC Egyptian Organization for standardization and Quality Control (2010) Maximum permissible limits of some pollutants in food.
- Park DL (2002) Effect of processing on aflatoxin. Advances in Experimental Medicine and Biology 504: 173-179.
- Rastegar H, Shoeibi S, Yazdanpanah H, Amirahmadi M, Khaneghah AM, et al. (2016) Removal of aflatoxin B1 by roasting with lemon juice and/or citric acid in contaminated pistachio nuts. Food Control 71: 279-284.
- Topi D, Babic J, Jakovac Strajn B, Tavcar Kalcher G (2023) Incidence of aflatoxins and ochratoxin a in wheat and corn from Albania. Toxins 15(9): 567.
- Zuki Orozco BA, Batres Esquivel LE, Ortiz Perez MD, Juarez Flores BI, Diaz Barriga F (2018) Aflatoxins Contamination in Maize Products from Rural Communities in San Luis Potosi, Mexico. Ann Glob Health. 84(2): 300-305.






























